
Panel discussion on...
Healthy lifestyle

Scientific and regulatory progress for prebiotic inulin
Understanding the needs of today’s health-seeking consumers
In the past decades a tremendous interest has grown in the importance of one’s intestinal microbiota, i.e. the microbial community present in our gastrointestinal tract, in relation to health and well-being. Furthermore, methods to measure the microbiome have been increasingly developed and improved. These developments helped researchers to explore the microbiome better and study more and more members of the human gut microbiota and their functions. In this respect prebiotics play a crucial role in maintaining intestinal health as they serve as nourishment for beneficial gut bacteria, fostering a harmonious environment within our digestive system. Prebiotics are defined by ISAPP (International Scientific Association for Probiotics and Prebiotics) as ‘a substrate that is selectively utilized by host microorganisms conferring a health benefit’ (1). Selectivity means that the range of microorganisms affected must be limited, this could be one or several microbial groups, but not all. In addition, a prebiotic must show a health benefit that is scientifically proven and supported by well-controlled research trials. ISAPP recognizes inulin, fructo-oligosaccharides (FOS), and galacto-oligosaccharides (GOS) as prebiotics (1). Inulin and FOS are linked with β-(2-1) bonds, this is a specific bond that is not digested nor absorbed in the human digestive tract. Therefore, upon consumption, they reach the colon intact where bifidobacteria ferment them as energy source while increasing in number. This is called the bifidogenic effect. Bifidobacteria have been maintained exclusively within our ancestors for hundreds of thousands of generations (2). Bifidobacteria are carbohydrate-fermenting (saccharolytic) bacteria associated with beneficial effects such as maintenance of the gut barrier function, inhibiting pathogens and supporting immunity amongst many others (3). This bifidogenic effect of inulin and FOS is confirmed in all age groups, including adults and elderly (4, 5). Doses as low as 3 to 5 g of chicory inulin-type fructans per day can give a bifidogenic effect in adults as show by modern molecular technologies such as 16S rRNA sequencing or quantitative PCR (6). A claim for this effect and even for a prebiotic effect may be made on foods in many countries with conditions depending on the regulatory body. In very young children (6-24 months) a bifidogenic effect was already shown at 2 g/d (7).
Novel microbiome benefits
The number of human clinical studies investigating the potential for prebiotics in health, especially in the gut-brain axis for potential prevention or treatments for mental health and cognition has dramatically increased in the last years. Researchers are fascinated in the cross-talk between the gut and the brain, the so-called ‘gut-brain axis’, an intricate communication network connecting our gastrointestinal tract to our central nervous system. It was already known that the brain and the gut communicate with each other about swallowing, digestion, and satiety, but also stress and mood seem to be connected to the gut via the gut-brain axis. The microbiota in the colon ferment prebiotic fibers or complex carbohydrates to short chain fatty acids, like butyrate, acetate, and propionate, which support our health (8). The idea that the gut microbiota is associated with and may influence mood disorders started when researchers observed a high prevalence of anxiety and depression disorders in people with gut disorders who often have a disbalanced microbiome. One way of restoring or retaining a healthy microbiome is via diet. Research has shown that prebiotic supplementation may reduce stress responsiveness, anxiety, and depressive-like behavior (9). For example, a few studies have been conducted using inulin and/or oligofructose and showed an improved Mental Health score, mood and episodic memory after ingestion (10, 11). While clearly more human studies are necessary to further explore these associations, the role of prebiotics to positively impact the human gut microbiome has been thoroughly established.
Inulin is a proven plant-based prebiotic
Due to the link of the gut microbiome with health effects, the prebiotic claim is becoming increasingly recognized by consumers. Chicory inulin adheres to the criteria of ISAPP and is currently the only plant-based prebiotic endorsed by ISAPP (1). As described above it has a selective effect on the human gut microbiota of infants to adults to elderly especially increasing the abundance of bifidobacteria as demonstrated by systematic reviews and meta-analyses of numerous human clinical studies (4, 12, 13). A few other genera such as Lactobacillus, Anaerostipes, or Faecalibacterium are also occasionally observed to be increased in abundance and are associated with the metabolic conversion of lactate and acetate produced by bifidobacteria to butyrate (14, 15, 16). These short chain fatty acids are implicated in the many health benefits of inulin (17).
In some countries, health claims may be made on the property of inulin to increase bifidobacteria in the intestine. For example, the functional claim “Prebiotic promotes the growth of good Bifidus bacteria to help maintain a healthy digestive system” can be made in Singapore, provided that the exact identity of the prebiotic is declared on the product label and the food manufacturer/importer can ensure that the amount of prebiotic present in the product can confer the claimed effect. In Thailand there is an approved proprietary health claim statement for inulin, oligofructose and short-chain inulin from chicory roots “inulin/ fructo-oligosaccharide / oligofructose helps to increase bifidobacteria in the intestine” which require specific serving sizes (18). The claim has certain conditions of use, including particular product types, and the food manufacturer must submit the product information to the Thai FDA to confirm all is in accordance with these conditions.
Most importantly, besides the groundbreaking novel research on prebiotics impact on mental health and cognition, inulin has well-established scientifically proven beneficial health effects such as improving digestive health based on numerous human clinical studies (19, 20, 21, 22). In the EU, there is an authorized health claim for chicory inulin for improved stool frequency (23). Thus, numerous randomized controlled clinical trials support both the selective gut microbiota effect and health benefit of inulin.
Prebiotic & intestinal bifido health claims
As inulin conforms to all the ISAPP prebiotic definition criteria, the term prebiotic may be used in different countries on food labels provided various country regulation-specific conditions are met. In the United States, for example, ‘prebiotic’ may be made on product labels by food manufacturers. This is considered a structure-function claim which is allowed provided there is sufficient substantiation in the form of a dossier developed by the manufacturer making the claim.
Health claims on foods in the European Union are regulated by the EU Regulation on Nutrition and Health Claims Made on Food (24). Based on the advice of the European Food Safety Authority (EFSA), the European Commission states that the claim “contains prebiotic” is considered an implied general health claim. These general health claims or Article 10(3) messages can be used if they are accompanied by a specific health claim authorized under Articles 13 or 14. EFSA described a prebiotic effect as “referring to aspects related to increasing numbers of bacteria that are considered to be beneficial” (25). As mentioned above, there is an authorized health claim for chicory inulin for improved stool frequency (23). In the scientific opinion EFSA acknowledged that native chicory inulin could exert an effect on stool frequency by stimulating bacterial growth in the gut and by increasing bacterial cell mass, thus linking inulin, the gut microbiota, and the bowel habit benefit. Consequently, a message such as ‘inulin is a prebiotic’ may be made in conjunction with the authorized inulin bowel habit health claim provided all other conditions of use are met.
Currently certain European countries have initiatives to use the term prebiotic for example in Italy for certain prebiotics such as inulin and FOS (26).
The strong and solid scientific evidence for chicory inulin in terms of impact on the microbiota and substantiated health benefits is essential supporting such regulatory initiatives.
Perspectives
Inulin is a clinically proven prebiotic with substantial scientific data based on more than 20 years of research especially gently maintaining bowel regularity due to balancing the composition of the gut microbiome. This strong science has consequently generated a variety of health claims globally for bifidogenic or improved microbiota, to improved digestive health and prebiotic, depending on the country/region. In the EU, inulin has an authorized health claim for maintaining bowel habit, besides blood glucose lowering. Remarkably a recent study showed intake of prebiotic inulin in healthy elderly twins supported improved cognition (27). Such emerging science in the field of prebiotic and cognition considering current aging populations is an exciting development.
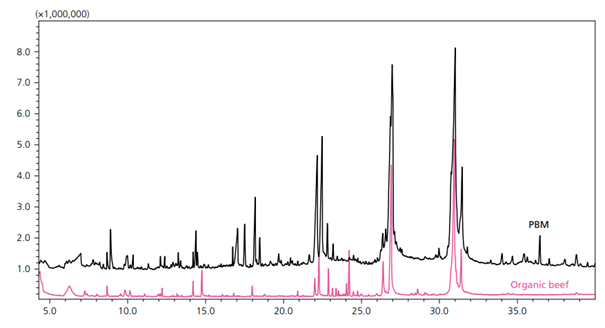
Figure 1. Overlaid Representative Chromatograms for PBM (black) and Organic Beef (pink) (6).
Panelists
References and notes
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews Gastroenterology and Hepatology. 2017;14(8):491-502.
- Moeller AH, Caro-Quintero A, Mjungu D, Georgiev AV, Lonsdorf EV, Muller MN, et al. Cospeciation of gut microbiota with hominids. Science. 2016;353(6297):380-2.
- Alessandri G, Ossiprandi MC, MacSharry J, van Sinderen D, Ventura M. Bifidobacterial Dialogue With Its Human Host and Consequent Modulation Of The Immune System. Front Immunol. 2019;10.
- Swanson KS, de Vos WM, Martens EC, Gilbert JA, Menon RS, Soto-Vaca A, et al. Effect of fructans, prebiotics and fibres on the human gut microbiome assessed by 16S rRNA-based approaches: a review. Benef Microbes. 2020:1-30.
- Schaafsma G, Slavin JL. Significance of inulin fructans in the human diet. Comprehensive Reviews in Food Science and Food Safety. 2015;14(1):37-47.
- Reimer RA, Soto-Vaca A, Nicolucci AC, Mayengbam S, Park H, Madsen KL, et al. Effect of chicory inulin-type fructan–containing snack bars on the human gut microbiota in low dietary fiber consumers in a randomized crossover trial. The American Journal of Clinical Nutrition. 2020.
- Waligora-Dupriet A, Campeotto F, Nicolis I, Bonet A, Soulaines P, Dupont C, Butel M. Effect of oligofructose supplementation on gut microflora and well-being in young children attending a day care centre. International Journal of Food Microbiology. 2007;113(1):108-13.
- Marchesi JR, Adams DH, Fava F, Hermes GDA, Hirschfield GM, Hold G, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330-9.
- Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99:1877-2013.
- Reimer R, Willis HJ, Tunnicliffe JM, Park H, Madsen KL, Soto-Vaca A. Inulin-type fructans and whey protein both modulate appetite but only fructans alter gut microbiota in adults with overweight/obesity: A randomized controlled trial. Molecular Nutrition and Food Research. 2017;61(11).
- Smith AP, Sutherland D, Hewlett P. An Investigation of the Acute Effects of Oligofructose-Enriched Inulin on Subjective Wellbeing, Mood and Cognitive Performance. Nutrients. 2015;7(11):8887-96.
- Firmansyah A, Chongviriyaphan N, Dillon Drupadi H, Khan Nguyen C, Morita T, Tontisirin K, et al. Fructans in the first 1000 days of life and beyond, and for pregnancy. Asia Pacific journal of clinical nutrition. 2016;25(4):652-75.
- Nagy DU, Sándor-Bajusz KA, Bódy B, Decsi T, van Harsselaar J, Theis S, Lohner S. Effect of chicory-derived inulin-type fructans on abundance of Bifidobacterium and on bowel function: a systematic review with meta-analyses. Critical Reviews in Food Science and Nutrition. 2022.
- Wegh CAM, Puhlmann ML, Dam V, Doolan A, Meyer D, Vaughan EE, et al. A randomized, double-blind, placebo-controlled study to evaluate the effects of inulin on gut intestinal microbiota and bowel habit in adults with functional constipation. . Thesis: From healthy defecation to functional constipation in children & adults A clinical and microbiological perspective Wageningen: Wageningen University; 2022.
- Vandeputte D, Falony G, Vieira-Silva S, Wang J, Sailer M, Theis S, et al. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut. 2017;66(11):1968-74.
- Kiewiet MBG, Elderman M, El Aidy S, Burgerhof JGM, Visser H, Vaughan EE, et al. Flexibility of Gut Microbiota in Ageing Individuals during Dietary Fiber Long-Chain Inulin Intake. Mol Nutr Food Res. 2021;65.
- Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, et al. Short chain fatty acids in human gut and metabolic health. Benefical Microbes. 2020;11(5):411-55.
- Thai FDA letter: Approval for Health claim of Inulin products., MoPH 1010.4/17389 (2019).
- Dahl WJ, Wright AR, Specht GJ, Christman M, Mathews A, Meyer D, et al. Consuming foods with added oligofructose improves stool frequency: a randomised trial in healthy young adults. Journal of Nutritional Science. 2014;3(e7):doi:10.1017/jns.2014.6.
- Marteau P, Jacobs H, Baril B, Signoret C, Prevel JM. Effects of native chicory inulin on constipation in elderly people. Wageningen: Wageningen Academic Publishers; 2010 2010.
- Micka A, Siepelmeyer A, Holz A, Theis S, Schön C. Effect of consumption of chicory inulin on bowel function in healthy subjects with constipation: a randomized, double-blind, placebo-controlled trial. International journal of food sciences and nutrition. 2017;68(1):82-9.
- Watson AW, Houghton D, Avery PJ, Stewart C, Vaughan EE, Meyer PD, et al. Changes in stool frequency following chicory inulin consumption, and effects on stool consistency, quality of life and composition of gut microbiota. Food Hydrocolloids. 2019;96:688-98.
- Scientific Opinion on the substantiation of a health claim related to "native chicory inulin" and maintenance of normal defecation by increasing stool frequency pursuant to Article 13.5 of Regulation (EC) No 1924/2006. EFSA Journal. 2015;13(1):3951.
- Commission E. Regulation (EC) no 1924/2006 of the European parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Official Journal of the European Union. 2006;30 December 2006:L404.
- Scientific Opinion on the substantiation of health claims related to various food(s)/food constituents(s) and increasing numbers of gastro-intestinal microorganisms (ID 760, 761, 779, 780, 779, 1905), and decreasing potentially pathogenic gastro-intestinal microorganisms (ID 760, 761, 779, 780, 779, 1905) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010;8(10):1809.
- Direzione Generale Per L'Igiene E la Sicurezza Degla Alimenti E la Nutrizione - Ufficio n 4. Guidelines on Probiotics and Prebiotics. Ufficio 4 (2018).
- Ni Lochlain M, Bowyer RCE, Moll JM, Garcia Paz M, Wadge S, Baleanu AF, et al. Effect of gut microbiome modulation on muscle function and cognition: the PROMOTe randomised controlled trial. Nature Communications. 2024; 15:1859.
Questions
1.
2.
3.
4.
5.
How have consumer awareness & demands related to healthy lifestyle changed in the last 12 months?
Where do you see the greatest scientific achievement in nutritional sciences in the last 12 months?
Are there specific health benefit areas in which significant more clinical studies are being done than in others, comparing the last 3 years?
What are the key influencers driving consumer purchasing decision for supplements or health foods? Is substantiation of claims important?
How do consumer today judge their health status?
Please put the following parameter in order 1 highest priority 7 lowest priority:
- Physical Symptoms, like pain, fatigue, constipation, weight gain
- Fitness Levels: endurance, strength, flexibility,
- Health Tracking Devices and heart rate, sleep patterns, steps taken
- Diet and Nutrition: intake of fruits, vegetables, whole grains, proteins.
- Mental and Emotional Well-being: stress levels, emotional balance, happiness
- Quality of sleep: Sleep quality and duration
- Medical Check-ups: blood pressure checks, cholesterol screenings, blood sugar tests
6.
Delivery formats can support consumer compliance and underline the technology driven approach of the brand.
a.
b.
c.
Do you see a consumer trend in preferences for certain delivery formats?
Are delivery format preference a regional, cultural aspect like taste?
Do you see a trend to carry out ingredient clinical trials using a selected delivery format as study product formulation or are most clinical studies still done in capsules?
7.
Consumer health concerns can vary widely across different regions and demographics globally. However, several common health concerns tend to be prevalent across various countries and populations due to globalization, lifestyle changes, environmental factors, and access to information.
a.
b.
Which are the key global consumer health concerns?
Do you see regional differences?
8.
There is a significant increase in the availability of apps and digital platforms focused on healthy lifestyle, particularly to support personalized approaches to mental wellbeing, metabolic support, weight management and physical fitness.
a.
b.
c.
d.
Do you see lifestyle apps as competition for supplement brands?
Do you think that these apps help to educate consumers, being more targeted when searching for supplements?
Did you consider setting up a lifestyle app to promote your supplement or your ingredient?
Did you set up a lifestyle app to promote your supplement/ ingredient and can you explore about your experience and business impact?
9.
AI (Artificial Intelligence) algorithms are today offered for various aspects of clinical trials to proof efficacy of your health ingredient or supplement.
a.
b.
c.
d.
Are consumers looking for substantiation of claims through AI driven clinical trials?
Did you consider working with an CRO specialist in AI to investigate your health ingredient or supplement?
If you applied already AI methods during the discovers/ development of your health ingredient, please share your experience and recommendation with us.
Do you think that in 2030 AI will be a manifest tool for clinical trials?
10.
A healthy lifestyle may also involve a commitment to sustainable practices for both personal and planetary health. This could include supporting ethical and environmentally conscious products.
a.
b.
c.
d.
e.
Do you agree that sustainability has become a growing concern also in the nutraceutical industry?
Are consumer looking proactively for brands which have ethical and environmental principles?
Are you looking for ethically sourced raw materials, ingredients?
Did you implement measures to establish resource-efficient processes?
Are you developing socially useful products and how do you define it?
11.
What are the major geographical differences related to healthy lifestyle trends?
a.
b.
c.
d.
e.
What are the latest trends related to claims and product forms in China?
In the U.S.
In Mexico
How are claims and dosage forms differentiated in Europe? How does your company ensure compliance with health and safety regulations in Europe?
Do you have a country where you would like to speak about trends for products?
12.
Focus clinical studies. Overall, there has been a recognized push for more gender-inclusive, geographical, and target group focused clinical research. The same trend can be seen in clinical trials for supplements or functional foods.
a.
b.
c.
d.
Please select an area you are active in and let us know which is your hot topic ingredients for 2024 for this area, based on substantiated claim, parameters, biomarkers being studied and/ or clinical study being done. Please name ingredients by scientific name and composition and not by brand name.
Please select your geographical area:
Western (EU, USA)
Asia (China, Japan, Asia Pacific)
Americas (Middle and Latin America)
Kids development/ early life nutrition/infant in Western countries and AP
Adult nutrition and prevention of developing disease
Elderly nutrition and how to support quality of life during aging
Sports nutrition, within different life decades and activity levels
References and notes
























