KEYWORDS
Pectin
Plant-based gummies
Gummy formulation challenges
Nutraceuticals
Front-of-pack nutrition labelling
The following article discusses various impacting factors based on my perspective. It is important to note that the information provided is subjective and should be treated as an opinion rather than objective fact. The gummy market is experiencing rapid growth, capturing the attention of dietary supplement makers and brands who are keen to invest in gummy production technology. As the dietary supplement industry shifts toward cleaner labels and vegan choices, gummy manufacturers are increasingly exploring formulations that utilize plant-based hydrocolloids, particularly pectin. However, it is important to note that pectin has distinct gelation requirements compared to gelatin which can lead to formulation and manufacturing challenges. This article will overview the basic chemistries of pectin, focusing mainly on factors in which pectin manufacturers control and factors in which formulators control. By understanding and applying the knowledge of these factors, formulators are able to understand the impact of their formulation choices and overcome technical challenges often associated with making pectin gummies.
Abstract
Pectin gummies in the nutraceutical market: navigating formulation challenges
Driven by consumer preferences for convenience and indulgence, gummies are the fastest-growing segment in the dietary supplement market. The global market is projected to grow with a compound annual growth rate CAGR(2023 -2033) of 13.4%, and is estimated to reach a market value of $99.4 billion in 2033 (1). In the U.S. dietary supplement market, nearly one in four dollars is spent on gummies (2). This reflects consumer preference for this great-tasting format. To meet consumer preferences, the market continues to shift toward gummies, as evidenced by the number of new gummy supplement products entering the global market, which is growing at a CAGR(2018-2023) of 27%, more than twice as fast as the overall category (3).
Consumers see plant-based products as healthier and better for the planet. This is also reflected in the declining share of new gelatin-based gummies entering the market. While nearly 60% were gelatin-based in 2018, this number decreased to 32% in 2023. Pectin has become the most widely used alternative to gelatin. In 2023, more than 55% of new gummies contained pectin as the sole hydrocolloid or blended with other plant-based gelling agents. As manufacturers invest in the technology to incorporate gummy production into their services, formulators are faced with the difficult task of learning how to make a gummy. Unlike confectionery gummies, the inclusion of nutraceutical ingredients poses additional challenges in the ability of the hydrocolloid system to form a gel network, especially when using plant-based hydrocolloids. Pectin and other plant-based hydrocolloids can be challenging to work with, requiring careful consideration for formulation and processing. The gelation requirements of pectin typically used in gummies can create added stressors for formulators since these gummies cannot be melted down and reworked. Understanding both the internal factors (controlled by manufacturers of pectin) and external factors (factors formulators control through application) can better guide formulators in creating successful gummy formulations with pectin.
Understanding pectin at the molecular level
- Classification of pectin and requirements for gelation
Pectin, a fascinating polysaccharide, plays a crucial role in gummy formulations. Pectin is divided into two classifications known as high ester (HE) or high methoxy (HM) pectin and low ester (LE) or low methoxy (LM) pectin; low ester pectin can be further classified into low ester conventional (LC) and low ester amidated (LA). HE pectin requires high soluble solid concentration and low pH to facilitate gelation. LE pectin requires the presence of calcium to act as a bridge between pectin molecules (4). For most gummy applications, HE pectin is typically used as it provides firmer textures and enables rapid demolding. However, some nutraceutical ingredients could benefit from using LE pectin as well.
- Understanding the building blocks of pectin
Pectin is essentially a linear molecule comprised of smooth regions where galacturonic acid molecules are present and hairy regions that consist of rhamnose molecules with branching neutral sugars. The polymer's functionality comes from esterifying the carboxylic acid groups on the galacturonic acid units with methanol. The number of esterified groups on the pectin chain determines the gelation requirements. Specifically, for HE pectin, when pH is above 3.5, approximately 50% of the carboxylic acid groups will be negatively charged, creating repulsion between the pectin chains (5). One aspect of HE pectin’s binding capability is determined by the carboxylic acid’s negative charge, the other at the hydroxyl groups on the acid chain and lastly at the methyl groups.
- Requirements of HE pectin gelation
HE pectin requires the presence of a co-solute (i.e., sugar) and low pH before it forms a gel. The gel is stabilized by junction zones formed by a combination of hydrogen bonding and hydrophobic interactions. The pH must be low enough (typically below 3.5) to suppress the repulsion between pectin chains caused by the negatively charged carboxylic groups and the solids must be high enough to reduce the water activity in the system to promote hydrophobic interaction among the methyl groups. Approximately one third of HE pectin’s gel formation potential is reliant on hydrophobic interactions; the remainder is from hydrogen bonding (6). The distribution of the ester groups on the chain influences pectin’s ability to participate in both types of interactions.
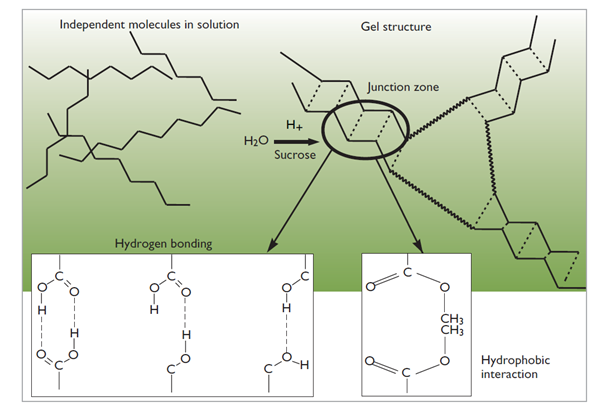
Infographic to describing HE pectin’s gelling mechanism (11).
Factors that influence HE pectin’s gelation requirements, which are carefully managed through pectin manufacturers
Pectin’s functionality and gelation requirements are intricately tied to the extraction and processing methods employed by suppliers. Consequently, pectin manufacturers wield control over critical factors such as the degree of esterification, the molecular weight, and the concentration of the block configuration created by the neutral sugars. These three factors determine the requirements for promotion of gelation, gel strength, reactivity to calcium and the gel setting time. The degree of esterification controls how rapidly and firmly a will set due to the higher concentration of methylated ester groups being present on the pectin chain, allowing for more junction zones to be formed. Formulations that tend to have pregelation problems can benefit from extra slow and slow set pectin grades which typically have a degree of esterification of 50-70%. Block structures on the pectin chain dictates how much interaction pectin will have with minerals such as calcium, which results in ionic bridging of the pectin chains, this type of interaction will increase the setting temperature requirement thus result in more likelihood of pregelation. While formulators may not directly manipulate these factors, understanding their impact on pectin’s functionality will enable formulators to make more sound formulation and processing decisions through the factors they have control over such as soluble solids, pH, and buffer salt systems.
Factors that formulators can control
- Soluble solids concentration
External factors such as soluble solids concentration (%SS), pH, type of co-solutes and buffer salt system are controllable by the formulator. Each of these factors has a tremendous effect on gelation and texture. Typically, the higher the soluble solids content, the higher the setting temperature requirement will be, and the result will be a firmer gummy. This is related to the reduction of the water activity in the gel system to promote hydrophobic interactions, the increased number of hydrophobic interactions reduces setting time and aids in gel network stability (7). However, beyond the upper limit (~82%), gelation occurs too rapidly, therefore requiring too high of a deposit temperature. This is typically not feasible for production and can often result in pregelation.
- Co-solute system
The role of the co-solute system is drastically related to %SS; as the co-solute system is responsible for controlling the water activity in the system. Each sugar or sugar replacement typically used in the nutraceutical industry has its own affinity for water and calcium ions that are naturally present in the gel system. Typically, lower molecular weight carbohydrates have very little effect on gel structure and strength. The same cannot be said for molecules with higher molecular weights, these molecules can sterically interfere with the pectin chain’s ability to create hydrogen bonds with one another (8).
- Potential of hydrogen (pH)
In addition to soluble solids concentration, pH is an important factor that the formulator can control. Pectin has an optimal threshold for gel strength as it relates to pH. To effectively allow the formation of junction zones between pectin chains, the negative charge caused by the carboxylic acid groups must be neutralized through the addition of acid (9). Pectin, in general has a gelation range around 2.8-3.6 pH, however, some pectin manufacturers have been able to modify this range slightly through their extraction and processing methods yielding ranges ending a little higher than 3.6 but less than 4.0. There is an optimal pH range where the highest gel strengths can be achieved, working on either end of the pH ranges will typically give weaker gel strength. If you have too low of a pH and gelation is likely occurring too rapidly for a cohesive network to be formed and increasing the chances of pregelation; too high of a pH and the pectin chains are not sufficiently neutralized to promote strong and or adequate number of junction zones to stabilize the gel network.
- Buffer-salts
Lastly, buffer salts are an important and crucial part of pectin gummy formulations as they help control the gelation rate and setting temperature requirements. Each buffer salt’s counter ions and complex directly interacts with the pectin in the system. The counter ion, depending on molecule size, can participate in ionic bridging of the pectin molecules, helping with stabilization of junction zones. The complexing agent has direct impact through its ability to sequester calcium ions present in the system, resulting in less ions to help with bridging of pectin chains(10). It is critical to understand both the ion and complexing agent’s ability to interact with ingredients present in the gel system to balance the negative and positive effects it has in gel formation.

Summary
By comprehending the internal factors managed by manufacturers and their influence on external gelation requirements, formulators gain a strategic advantage. Understanding these critical factors empowers formulators to assess the impact of formulation choices on texture, setting temperature, gelling potential, gelling rate, and the likelihood of pregelation. Moreover, this knowledge proves invaluable during troubleshooting and addressing formulation challenges in production.
Conclusion
With the recent rise in vegetarian eaters across the globe, new VFGs are required to provide guidance to ensure that daily nutritional needs are met. Using these guidelines, professional and amateur vegetarian athletes have the opportunity to enhance their performance by tailoring their diets based on sport-specific needs. With new food guides such as VegPlate, and now VegPlate for Sport, athletes and sport nutritionists alike will be better equipped to create effective meal programs using evidence-based resources.
References and notes
- Choudhury, N.R., Nutraceuticals Gummies Market Outlook 2023 to 2033. Future Market Insights Report. 2023. https://www.futuremarketinsights.com/reports/nutraceutical-gummies-market
- Delivery Format Report 2023. Nutrition Business Journal. 2023, 31-37. https://store.newhope.com/products/2023-delivery-format-report
- Chychula, A. Vitamins, Minerals & Supplements-US-2023. Mintel GNPD. 2023. https://clients.mintel.com/download/brochure/vitamins-minerals-supplements-us-2023
- Thakur, B. R., Singh, R. K., Handa, A. K., & Rao, M. A., Chemistry and uses of pectin — A review, 1997, Critical Reviews in Food Science and Nutrition, 37(1), 47–73. https://doi.org/10.1080/10408399709527767
- Celus, M., Kyomugasho, C., Van Loey, A.M., Grauwet, T. and Hendrickx, M.E., Influence of Pectin Structural Properties on Interactions with Divalent Cations and Its Associated Functionalities, 2018, Comprehensive Reviews in Food Science and Food Safety, 17: 1576-1594. https://doi.org/10.1111/1541-4337.12394
- El-Nawawi, S.A., Heikel, Y.A., Factors affecting gelation of high-ester citrus pectin. 1997, Process Biochemistry, 32: 381-385. https://doi.org/10.1016/S0032-9592(96)00076-3
- Bulone, D. Giacomazza, D., Manno, M., Martorana, V., San Giagio, P.L., Sucrose pectin interactions from solutions to gels, 2010, Food Hydrocolloids, 226-240. https://www.researchgate.net/publication/215638279_Sucrose_pectin_interaction_from_solution_to_gels
- Morris, E.R., Richardson, R.K., Tsoga, A., Role of cosolutes in gelation of high methoxy pectin. Part 1. Comparison of sugars and polyols, 2004, Food Hydrocollods, 18:907-919. https://doi.org/10.1016/j.foodhyd.2004.03.001
- Harvey, H.G., The mechanism of pectin jelly formation with respect to pH conditions, with particular reference to setting temperature phenomena., 1950, Journal of Science, Food Agricultrure, 1: 307-311. https://doi.org/10.1002/jsfa.2740011005
- Pectin User Guide, Danisco, 2009, 8: 1-5
- Introduction to Grindsted® Pectin, Danisco technical memorandum no.TM 8-2e,1-4.

