Biotics
Applications of functional ingredients in different product categories:
the example of probiotics
Irina Spacova1* Arthur C. Ouwehand9*
Sylvie Binda2, Jessica A. Younes3, Solange Henoud2, Sophie Legrain-Raspaud4,
James Dekker5, Jordi Espadaler-Mazo6, Philippe Langella7, Rebeca Martín7, Marco Pane8
*Corresponding authors
1. Research Group Environmental Ecology and Applied Microbiology, Department of Bioscience Engineering, University of Antwerp, Antwerp, Belgium
2. Rosell Institute for Microbiome and Probiotics, Montreal, Canada
3. International Probiotics Association, California, USA
4. Gnosis by Lesaffre, Marcq-en-Barœul, France
5. Fonterra Research and Development Centre Co., Ltd., Palmerston North, New Zealand
6. R&D Department, AB-Biotics SA (Kaneka Group), Barcelona, Spain
7. Université Paris-Saclay, INRAE, AgroParisTech, Micalis Institute, Jouy-en-Josas, France
8. Probiotical Research, Novara, Italy
9. IFF Health, Kantvik, Finland
Irina Spacova1*
Arthur C. Ouwehand9*
Sylvie Binda2, Jessica A. Younes3,
Solange Henoud2, Sophie Legrain-Raspaud4,
James Dekker5, Jordi Espadaler-Mazo6, Philippe Langella7, Rebeca Martín7,
Marco Pane8
*Corresponding authors
1. Research Group Environmental Ecology and Applied Microbiology, Department of Bioscience Engineering, University of Antwerp, Antwerp, Belgium
2. Rosell Institute for Microbiome and Probiotics, Montreal, Canada
3. International Probiotics Association, California, USA
4. Gnosis by Lesaffre, Marcq-en-Barœul, France
5. Fonterra Research and Development Centre Co., Ltd., Palmerston North, New Zealand
6. R&D Department, AB-Biotics SA (Kaneka Group), Barcelona, Spain
7. Université Paris-Saclay, INRAE, AgroParisTech, Micalis Institute, Jouy-en-Josas, France
8. Probiotical Research, Novara, Italy
9. IFF Health & Biosciences, Kantvik, Finland


KEYWORDS
Probiotics
Functional foods
Dietary supplements
Live biotherapeutics
Regulation
Manufacturing
Abstract
How probiotics as functional ingredients are incorporated in food, dietary supplements, and live biotherapeutic products depends on and is influenced by regulations, manufacturing, product development, and clinical research. Here, we discuss the challenges and opportunities that exist within these categories, depending on the intended use, target population, and market region of the products. Aspects related to safety, efficacy, and quality of probiotic products, as well as the preclinical and clinical research requirements for each category are discussed. Finally, we discuss the potential economic benefits and reimbursement schemes of probiotic products, and the need for harmonization of regulatory frameworks and standards.
Introduction
Functional ingredients can be utilised in three main categories of products: food, dietary supplements, and drugs. Each of these categories not only has its own regulation, but also their own requirements for manufacturing, product development, and preclinical and clinical research. Probiotics can be classified into either of these three main regulatory categories, namely as probiotic foods (PF), probiotic dietary supplements (PDS), and live biotherapeutic products (LBPs). Hence, taking probiotics as an example, we will discuss the challenges and opportunities that exist within these categories. PF and PDS are intended to maintain, support, or enhance health in healthy or at-risk populations, while LBPs are pharmaceutical/medicinal products intended to prevent, treat, or cure diseases (1).
Regulations and approval processes vary based on the intended use and regulatory category. PF and PDS usually target healthy populations and have less stringent regulations, depending on their product nature and the region. In contrast, LBPs, being drugs, have more stringent regulation, safety, efficacy, and quality criteria for marketing authorization. However, due to their living nature and multifactorial modes of action, the regulatory landscape is more complex than that of conventional drugs. Developing a new probiotic product requires meeting different quality requirements for various global markets. Costs vary depending on the category, with LBPs generally incurring higher expenses than PF and PDS due to the longer development processes and more extensive clinical trial requirements (2) (Figure 1).
This review is a condensed version of a comprehensive paper published recently (3) and aims to provide insights into what can be expected from food, dietary supplements and drugs as finished products using probiotics as an example, and how regulations impact their development, manufacturing, and clinical research. This review covers orally administered probiotics for human applications and excludes medical devices and topical cosmetic products. While these concepts can be extended to animals, this review solely covers human applications.
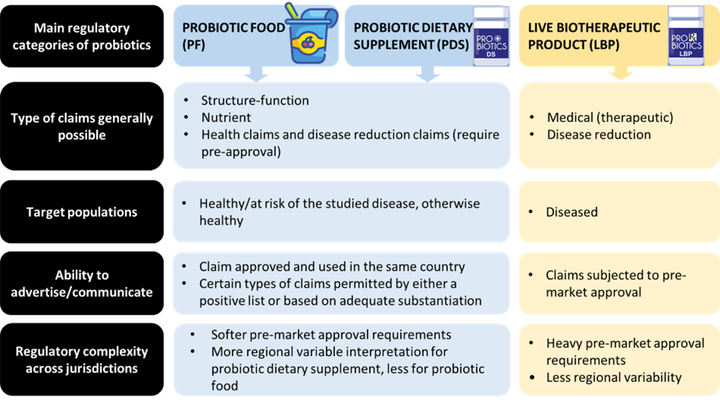
Figure 1. Summary of major regulatory classes of probiotics. It should be noted that PF and PDS can also be used in a person with a disease to promote health for the same reasons as in a person without a disease, but here the intended population is highlighted from a regulatory standpoint. Modified after (3).
Regulation
Probiotic products are subject to diverse regulations depending on their intended use, leading to different regulatory categories: PF, PDS, or LBPs. Intended use is determined by factors such as the product's nature (e.g., ingredient, form) and its purpose (e.g., supporting bodily functions or treating diseases). The regulatory framework of the chosen market needs also to be considered. PF and PDS are generally governed by food law in most countries, but specific regulations for PDS have been established in some regions, outlining expectations on safety, efficacy, and quality. Certain countries, such as South Africa and Australia, may even regulate specific PDS as drugs due to their formulations or health claims (4).
Some regions employ positive lists of specific probiotic organisms that can be sold under pre-established safety conditions without the need for further pre-marketing approvals unless the intended use deviates from proscribed boundaries. For example, the European Union (EU) follows the qualified presumption of safety (QPS) list to regulate microorganisms (on species level) introduced into the food and feed chain, including PF and PDS (5). China has a similar list of organisms (on species level) that are allowed for use in food production and processing. However, for infants and toddlers a positive list on strain level is used in China. The USA has its generally recognised as safe (GRAS) regulation to indicate the safety of ingredients, including microorganisms. Note that while QPS regulates on (sub-) species level, GRAS specifies the microbial strain, dose, and its uses (6).
When probiotic products are intended for therapeutic use, such as treating or preventing diseases or conditions, they are generally classified as drugs and must therefore undergo a lengthy pre-marketing approval process. This is the case for LBPs, which are designed to target specific health conditions and necessitate extensive clinical trials to obtain regulatory approval.
Access to the market for PF and PDS can vary among regions. In Europe, probiotic products can quickly reach the market if they contain organisms that are included in the QPS list. However, obtaining approval for health claims of probiotic products has remained highly challenging due to the difficulty in establishing cause-and-effect relationships. For instance, more than 400 health claim applications have been rejected by the European Food Safety Authority (EFSA), although one specific claim has been approved for Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in yogurt and improving lactose digestion in individuals with lactose maldigestion (7). For other functional ingredients, most notably vitamins and minerals, health claims have been approved (8). This highlights, not surprisingly, that the nature of the functional ingredient also plays a key role in the regulatory process.
Canada offers a distinct category for natural health products, allowing a wide range of claims for probiotic products, from health maintenance to risk reduction, and management of symptoms. However, the regulatory landscape for PFs and LBPs is less well defined in Canada compared to Europe or the USA (1).
In the USA, probiotic products are classified under dietary supplements and cannot make claims to treat, cure, or prevent disease, but structure/function claims can be made based on adequate substantiation. The regulatory framework in the USA is complex and differs significantly from countries like Canada and Mexico, where claims may or may not be made for PDS on a case-by-case basis (9).
In Brazil, probiotics are regulated by the Brazilian Health Regulatory Agency (ANVISA), which is responsible for establishing the criteria for the registration, production, and marketing of foods, dietary supplements, and drugs. ANVISA has established guidelines for the use of probiotics in food and dietary supplements, which include requirements for the labelling, safety, and efficacy of these products. To make health claims, companies must provide scientific evidence to support the efficacy and safety of their products, which must be evaluated and approved by ANVISA. The labelling of probiotics must include information about the microorganisms present, their concentration, and their health benefits.
In China, the regulation of probiotics falls under the jurisdiction of several government agencies, including the National Health Commission, the State Administration for Market Regulation (SAMR), and the China National Center for Food Safety Risk Assessment (CFSA). Probiotics used in food and dietary supplements must meet the safety and quality standards set by these agencies, and their labels may not include health claims. Health foods, however, can make claims from 27 categories. These label claims must be supported by scientific evidence and approved by the relevant authorities. The studies for these claims are typically conducted by research institutions, universities, or third-party organizations that have been authorized to do so by the CFSA and SAMR.
A summary of regulation of probiotics foods, probiotic dietary supplements and LBPs in selected jurisdictions is shown in the Table.
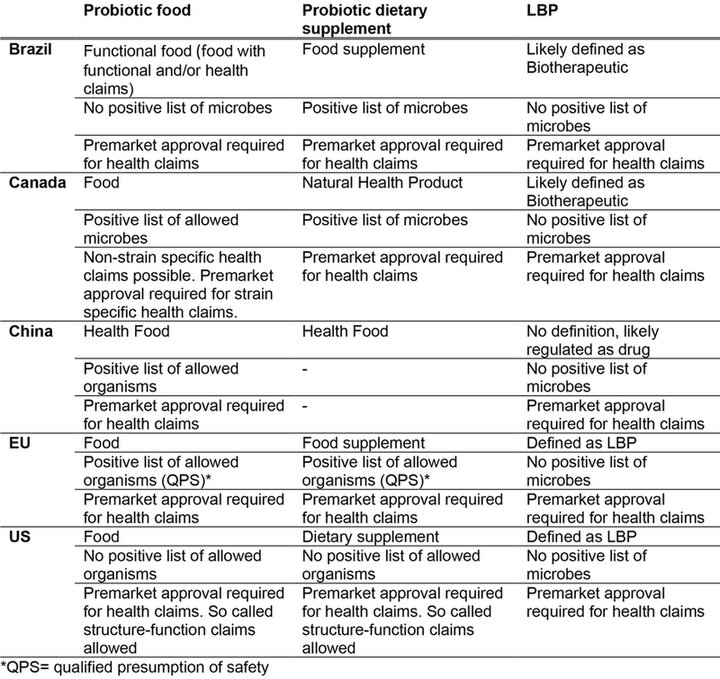
Table 1. Simplified comparison of regulation of probiotics foods, probiotic dietary supplements and live biotherapeutic products (LBP) in selected jurisdictions. From International Probiotics Association (IPA) internal data.
Manufacturing and production
To ensure appropriate, effective, and reproducible application of probiotic products in their intended regulatory category, well-controlled manufacturing processes and quality assurance procedures are required. While the safety of probiotic strains for healthy populations is well-established (10), this discussion focuses on quality and safety aspects of finished probiotic products. Manufacturing of PF, PDS, and LBPs begins with a culture seed stock, carefully prepared to contain a single pure strain, and verified free of contaminants through quality control testing. For products containing multiple strains, they are usually fermented as single strains before being formulated in the desired ratio. Although fermented probiotic foods may be produced with multiple strains simultaneously in the fermentation process.
Probiotic microorganisms have specific nutritional requirements, making the transition from lab to industrial conditions challenging. Careful selection and control of raw materials are essential, especially for microbes with strict growth conditions or anaerobic atmospheres. The industrialization process should be assessed alongside the probiotic strain's functionality to balance both aspects in manufacturing (11). To ensure safety, Hazard Analysis and Critical Control Points (HACCP) is a vital system used to identify and minimize food safety hazards during production. It focuses on prevention rather than relying solely on end-product testing. HACCP plans are developed to identify critical control points in the process, with monitoring and control measures to ensure their adherence (12). Additionally, Current Good Manufacturing Practices (cGMP) guidelines, provided by the FDA for drug production and quality control (13), play a crucial role in ensuring that products meet high standards of quality, safety, and efficacy. These guidelines involve proper design, monitoring, and control of manufacturing processes and facilities, along with the use of trained personnel and maintaining proper records.
As the risk of microbial contaminants in probiotics products may be higher compared to traditional drug products that can be subjected to sterilization, probiotic PF, PDS, and LBPs must adhere to specific quality control requirements. LBPs targeting vulnerable populations require more stringent control compared to PF and PDS, particularly regarding potential microbiological contaminants like Listeria monocytogenes and Bacillus cereus(14).
Manufacturing live microorganisms in PF, PDS, and LBPs can be subject to higher batch-to-batch variation, in contrast to most traditional chemically manufactured drugs. Efficient parametric, attributes and procedural controls during manufacturing are essential to retain reproducible health-promoting and/or disease-mitigating effects, with particular attention to factors influencing the probiotic mode-of-action (1). Hence, extensive, and detailed documentation is crucial throughout the manufacturing and quality control processes. Records must include observations, test results/raw data, and deviations. For detailed information on the production of probiotics we would like to refer to (11).
Product development
Product development with functional ingredients in various categories is heavily influenced by their intended use and corresponding regulatory framework. Early establishment of the intended use is essential during the product development stage. Regardless of the category, probiotic formulations typically undergo preclinical research before progressing to clinical trials to demonstrate health benefits and disease-mitigating effects in humans (15). Preclinical research involves in vitro, ex vivo, and/or in vivo testing which serves as a basis to support the intended use in the target population. Proper strain characterization, taxonomic assignment, and safety assessments are prerequisites for all probiotic products (16). However, the extent of preclinical data required may differ between PF/PDS and LBPs. Generally, more extensive preclinical data is required for LBPs compared to PF/PDS (17). During preclinical research, it is recommended to work as much as possible with research materials produced under commercial production conditions to ensure efficacy in the final product. Also, downstream processing should be managed carefully to preserve the structure and function of probiotic health effectors, such as surface components and enzymes (18). Developing a production process map can help identify the steps where changes could occur without compromising the desired output.
Testing the efficacy of the probiotic product in its final delivery matrix is ideal in preclinical tests, but it may be challenging to fully control development and production processes due to multiple mechanisms of action and effectors. Nevertheless, theoretical modelling approaches can be employed for well-studied probiotic strains with well understood metabolic processes that are relatively easy to follow and model. Additionally, compatibility and stability of probiotic combinations with other substances should be assessed during preclinical stages. Potential synergies may be modelled using metabolic mapping to enhance product efficacy (18). Apart from preclinical considerations, the time, effort, and costs of developing a novel probiotic product can vary significantly based on regulatory requirements, health claims, market value, and other parameters specific to each category. This information influences the decision on the probiotic product/functional ingredient category to target (4).
Clinical research strategies
Clinical research involving human intervention studies is essential for demonstrating the efficacy of probiotic products in maintaining human health (PF, PDS) or mitigating, preventing, or curing diseases (LBPs), thereby supporting specific health claims (19, 20). Clinical trials with functional ingredients across the three categories discussed here differ in design, evaluation criteria, and costs due to the intended use and final product format.
As discussed above, preclinical testing helps establish potential beneficial actions of a probiotic product, such as anti-pathogenic effects or immunomodulatory interactions, informing its potential as either a PF/PDS or LBPs. Although preclinical research may aid the design of subsequent clinical studies and assist in identifying relevant biomarkers, such studies alone cannot designate a microorganism as a probiotic (16). Clinical trials require different levels of evidence depending on the probiotic category. Randomized, double-blind, placebo-controlled trials provide strong clinical evidence for PF/PDS, especially in healthy populations, where beneficial effects must be demonstrated (21). However, identifying suitable biomarkers for risk reduction in healthy populations can be challenging, particularly for PF/PDS (22).
LBPs typically require more extensive supporting information than PF or PDS, including pharmacology, toxicology studies, and clinical protocols, before initiating clinical studies (23). The benefit-risk balance is a key parameter for evaluating LBPs, which may involve dose-response testing to measure drug efficacy (24). Although adverse event monitoring is required in all probiotic clinical trials, the severity of adverse effects in PF/PDS and LBPs is typically minimal and with low incidence. For PF/PDS, while investigation of tolerability issues like gastrointestinal discomfort are common, these alone may not be considered sufficient (25).
Reimbursement schemes are not currently available for PF and PDS but modelling studies have shown the potential economic benefits of probiotic products in the general population in various countries (France, USA, and Canada) (26). Reimbursement for LBPs follow country-specific general drug regulations, necessitating demonstration of better safety and medical benefits compared to standard care.
Conclusion
Functional ingredients have diverse applications and are regulated based on their intended use, resulting in distinct categories: foods, dietary supplements, or drugs. Ensuring quality and safety in probiotic products requires adherence to strict manufacturing processes and quality control standards regardless of category. Proper preclinical research and development planning are essential for demonstrating health benefits effectively and meeting market-specific requirements. Clinical research plays a vital role in establishing the efficacy of functional ingredients products, with trial designs tailored to each probiotic product category. Understanding regulatory frameworks and implementing harmonization efforts are crucial in navigating the complex landscape of functional ingredient regulations and advancing functional ingredients in general and probiotic applications in particular.
Disclosure
The work was conducted under the patronage of the International Probiotics Association (IPA, www.internationalprobiotics.org). SB, JAY, SH, SL-R, JD, JE-M, MP and ACO are employed by companies or organisations that manufacture, sell and/or promote probiotics. The other authors declare no conflict of interest. IS was supported by a postdoctoral grant (1277222N) from Research Foundation – Flanders (Fonds Wetenschappelijk Onderzoek; FWO).



References and notes
1. Cordaillat-Simmons M, Rouanet A, Pot B. Live biotherapeutic products: the importance of a defined regulatory framework. Exp Mol Med. 2020;52(9):1397-406.
2. EFSA Panel on Dietetic Products Nutrition & Allergies. Scientific and technical guidance for the preparation and presentation of a health claim application (Revision 3). EFSA J. 2021;19(3):e06554.
3. Spacova I, Binda S, ter Haar JA, Henoud S, Legrain-Raspaud S, Dekker J, et al. Comparing technology and regulatory landscape of probiotics as food, dietary supplements and live biotherapeutics. Front Microbiol. 2023;14.
4. Paraskevakos G. Global overview for probiotics: Trends, markets, and harmonization. Regulatory Focus. 2022(September):1-14.
5. EFSA BIOHAZ Panel, Koutsoumanis K, Allende A, Alvarez-Ordonez A, Bolton D, Bover-Cid S, et al. Updated List of QPS-Recommended Microorganisms for Safety Risk Assessments Carried Out by EFSA 2023. EFSA J. 2023;21(1): e07747.
6. Mattia A, Merker R. Regulation of probiotic substances as ingredients in foods: premarket approval or "generally recognized as safe" notification. Clin. Infect. Dis. 2008;46 Suppl 2:S115-8; discussion S44-51.
7. EFSA Panel on Dietetic Products Nutrition & Allergies. Scientific Opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010;8(10).
8. EFSA Panel on Dietetic Products Nutrition & Allergies. Scientific Opinion on the substantiation of health claims related to vitamins, minerals and omega‐3 fatty acids (ID 1, 2, 3, 4, 5, 7, 10, 11, 12, 111, 112, 168, 173, 174, 179, 184, 201, 210, 217, 362, 372, 717, 1464, 1515, 2872, 2874, 3094, 3095, 4279, 4280, 4281, 4282, 4284, 4285, 4286, 4287, 4289, 4291, 4292, 4708) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA Journal. 2011;9(4):2077.
9. FDA - United States Food and Drug Administration. Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information - Guidance for Industry. In: FDA, editor. Washington 2016.
10. Gueimonde M, Ouwehand AC, Salminen S. Safety of probiotics. Scand. J. Nutr. 2004;48(1):42-8.
11. Fenster K, Freeburg B, Hollard C, Wong C, Ronhave Laursen R, Ouwehand AC. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms. 2019;7(3):83.
12. Markel Services Inc. Hazard Analysis and Critical Control Points (HACCP); Understanding HACCP food safety standards: Markel Services Inc.,; 2023 [Available from: https://www.markelinsurance.com/resources/hazard-analysis-and-critical-control-points.
13. Food and Drug Administration. Facts About the Current Good Manufacturing Practices (CGMPs): FDA; 2021 [Available from: https://www.fda.gov/drugs/pharmaceutical-quality-resources/facts-about-current-good-manufacturing-practices-cgmps.
14. Merenstein D, Pot B, Leyer G, Ouwehand AC, Preidis GA, Elkins CA, et al. Emerging issues in probiotic safety: 2023 perspectives. Gut microbes. 2023;15(1):2185034.
15. Sanders ME, Merenstein DJ, Ouwehand AC, Reid G, Salminen S, Cabana MD, et al. Probiotic use in at-risk populations. J Am Pharm Assoc. 2016;56(6):680-6.
16. Binda S, Hill C, Johansen E, Obis D, Pot B, Sanders ME, et al. Criteria to Qualify Microorganisms as "Probiotic" in Foods and Dietary Supplements. Frontiers in microbiology. 2020;11:1662.
17. Rouanet A, Bolca S, Bru A, Claes I, Cvejic H, Girgis H, et al. Live Biotherapeutic Products, A Road Map for Safety Assessment. Front Med (Lausanne). 2020;7:237.
18. Lebeer S, Bron PA, Marco ML, Van Pijkeren JP, O'Connell Motherway M, Hill C, et al. Identification of probiotic effector molecules: present state and future perspectives. Current opinion in biotechnology. 2018;49:217-23.
19. EFSA Panel on Dietetic Products Nutrition & Allergies. Guidance on the scientific requirements for health claims related to the immune system, the gastointestinal tract and defence against pathogenic microorganisms. EFSA Journal. 2016;14(1):4369.
20. Food and Drug Administration. Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard. 2022.
21. EFSA. Outcome of a public consultation of the EFSA panel on dietatic products, nutrition and allergies (NDA) on the draft scientific guidance for stakeholders on health claim applications. EFSA Supporting publication. 2016;EN-986:1-98.
22. Quigley EMM. Clinical Trials of Probiotics in Patients With Irritable Bowel Syndrome: Some Points to Consider. Journal of neurogastroenterology and motility. 2022;28(2):204-11.
23. FDA, Investigational New Drug (IND) Application, (2022).
24. FDA. General considerations for clinical studies Guidance for industry. 2022. p. 31.
25. van der Geest AM, Schukking I, Brummer RJM, Pieterse H, van den Nieuwboer M, van de Burgwal LHM, et al. Inadequate safety reporting in the publications of randomised clinical trials in irritable bowel syndrome: drug versus probiotic interventions. Beneficial microbes. 2022;13(3):195-204.
26. Lenoir-Wijnkoop I, Merenstein D, Korchagina D, Broholm C, Sanders ME, Tancredi D. Probiotics Reduce Health Care Cost and Societal Impact of Flu-Like Respiratory Tract Infections in the USA: An Economic Modeling Study. Frontiers in pharmacology. 2019;10:980.


