Biotics
Postbiotics: A novel strategy for the leaky gut syndrome
Francesca Algieri1, Alessandra Silvestri1, Maria Rescigno2,3*
*Corresponding author
1. Postbiotica S.r.l, Milan, Italy
2. Humanitas University, Department of Biomedical Sciences, Pieve Emanuele, Milan, Italy
3. IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy

KEYWORDS
Postbiotics
Leaky gut
Tight junction
Epithelial barrier integrity
Microbiota
Gut vascular barrier
Abstract
The intestinal epithelial barrier (IEB) plays a pivotal role in maintaining homeostasis, acting at the frontline of defense against pathogen invasion and preserving the symbiotic relationship between commensal microbes and the host. Disruptions in the IEB, often reported as the leaky gut syndrome (LGS), contribute to various debilitating disorders, including inflammatory bowel disease (IBD), celiac disease, metabolic and neurodegenerative disorders. Existing approaches, such as probiotics and symbiotics, have shown inconsistent results, prompting a paradigm shift towards exploring the use of postbiotics—bioactive compounds released during microbial metabolism, which exhibit efficacy in eliciting specific host responses. The intricate interplay between IEB integrity and the microbiota underscores a critical role of postbiotics in preventing and reducing leaky gut conditions. Indeed, while live probiotics may represent a threat for the possibility of exacerbating mucosal inflammation and disseminating systemically, postbiotics offer a safer alternative due to their immunomodulatory properties, low toxicity and absence of live bacteria. This comprehensive exploration proposes the integration of well-characterized postbiotic components as a transformative strategy, particularly in chronic inflammatory conditions like IBD where conventional probiotics fall short. The enthusiasm surrounding postbiotics heralds a new era in therapeutic interventions, emphasizing their potential for re-establishing host microbe homeostasis and countering the challenges posed by the leaky gut syndrome.
Introduction
The human microbiome assumes a pivotal role in influencing human health and disease states (1). An increasing number of studies suggest that the beneficial effects associated with the establishment of a symbiotic gut microbiota are predominantly mediated by the metabolic by-products generated by microbial activity.
In 2012, the term “postbiotics” was coined to refer to “any soluble factor resulting from the metabolic processes of live bacteria or any released molecule capable of conferring health benefits through both direct and indirect mechanisms” (2). Since then, extensive research has been conducted on postbiotics and their effects, primarily dissecting their impact within the gut, but also on the skin. It is well-established that bacterial metabolites can cross the gut vascular barrier (3) and have been detected in the bloodstream (4) . Indeed, short-chain fatty acids (SCFAs), commonly produced by bacteria, have been detected in the blood (5) and their influence on inflammatory signaling pathways has been thoroughly investigated (4). Consequently, postbiotics hold promise as a therapeutic strategy for maintaining gut homeostasis.
The gastrointestinal tract serves as the primary interface between the body's internal environment and external surroundings(6). It comprises specialized barriers, such as the mucus layer, intestinal epithelial layer, and the lamina propria housing mucosal immune cells. These barriers dynamically receive and respond to various stimuli (7) .
Among them, the intestinal epithelial barrier (IEB) plays a crucial role in preventing harmful antigens and microorganisms from entering while facilitating the absorption of nutrients and water. Maintaining this delicate balance is essential for human well-being and is tightly regulated. The functionality of the IEB relies on intercellular junctions, namely the apical junctional complex, which includes tight junctions (TJs), adheres junctions (AJs), and desmosomes (8), (9).
TJs are a multi-protein complex, that are responsible for controlling the passage of antigens through the IEB and have a key role in maintaining barrier integrity. Various factors such as cytokines, gut microbiota, and dietary components influence intestinal TJs (10), (11).
Finally, the gut vascular barrier (GVB) is the deepest layer of the intestinal barrier. The GVB refers to a specialized structure within the gastrointestinal tract that regulates the passage of molecules, ions, and cells between the intestinal tissue and the bloodstream(12). When this barrier is compromised, endothelial cells can become damaged, and intestinal permeability can increase, allowing toxins, pathogens, and other harmful molecules to easily cross the intestinal mucosa and reach the bloodstream.
Recent researches have extensively demonstrated the IEB dysregulation can facilitate the passage of harmful substances through the intestine, which can then interact with the GVB favoring the occurrence of numerous intestinal and extra-intestinal disorders (13). In these conditions, dysfunction of the IEB leads to increased intestinal permeability, allowing antigens and microorganisms to enter the circulation and disseminate to distant organs (14). This process activates the immune system, triggering and sustaining chronic low-grade inflammation, typical of these disorders (15).
Given the link between intestinal permeability and numerous conditions, postbiotics can represent an innovative strategy to prevent or reduce intestinal permeability (Figure 1).
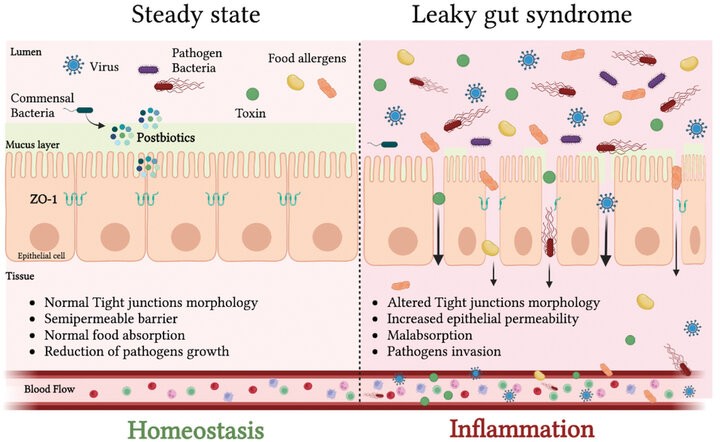
Figure 1. Intestinal epithelial barrier structure in homeostatic condition and inflammatory status (Adapted from Algieri et al. 2023 (16))
Leaky gut syndrome and current therapies
Description of the leaky gut syndrome and consequent pathologic conditions
The preservation of both epithelial (IEB) and endothelial (GVB) barrier function is essential to prevent various pathologic conditions, such as LGS, characterized by the passage of harmful agents, such as bacteria, toxins, and viruses, into the bloodstream(17). However, factors like stress, an unhealthy diet, alcohol, antibiotics, and certain medications can disrupt the balance of intestinal microbiota and compromise the integrity of the intestinal barrier, leading to increased intestinal permeability. LGS is characterized by chronic inflammation and altered tight junctions (TJs) in the intestine increasing intestinal hyperpermeability allowing harmful agents to cross the intestinal epithelium and enter the bloodstream, affecting various organs and systems. LGS and intestinal barrier dysfunction are associated with both intestinal diseases, such as inflammatory bowel disease (IBD) (17), (18), (19), encompassing Crohn’s disease (CD) and ulcerative colitis (UC), and irritable bowel syndrome (IBS) (20), celiac disease (21), (22) and extra-intestinal pathological conditions, including heart disease and metabolic disorders (obesity, type 2 diabetes and NAFLD/NASH (23), (24), (25)). Recently, also neurodegenerative disorders have been associated to the LGS (26), (27), (28), (29).
Consistently, IBS patients exhibit reduced levels of TJ proteins and elevated cytokine levels (20); patients affected by metabolic disorders (Obesity, type 2 Diabetes and NAFLD) display gut microbiota dysbiosis, microbial translocation and intestinal barrier disfunctions associated with changes in TJs integrity, and gut permeability(30) (31). Chronic heart failure (CHF) patients exhibit increased bowel permeability, leading to bacterial translocation and inflammation (32).
These findings underscore the critical role of intestinal permeability in various pathological conditions and highlight potential therapeutic targets for managing these diseases.
Microbiota-modulating therapeutic strategies to counteract the leaky gut
Reinforcing the intestinal barrier, in particular restoring the paracellular pathways stringency, mainly targeting TJs, has been suggested as a therapeutic strategy to restore intestinal homeostasis and prevent related-disorders.
Two major regulators of intestinal barrier have been identified in diet - prebiotics, and secondly within the intestinal microbiota – probiotics. Both players are related to lifestyle, suggesting that environmental factors might have an impact on intestinal barrier and gut health (33).
Nutritional interventions play a significant role in managing the leaky gut syndrome, with strategies focusing on avoiding sugar and fat-rich diets while incorporating elements like prebiotics and probiotics.
High-fiber intake has shown beneficial in various chronic diseases as fiber fermentation by gut microbiota produces valuable molecules like SCFAs, glutamine and vitamins among others, which play a crucial role in protecting intestinal barrier integrity (34), (35), (36). However, assumption of fibers is often associated with production of gas, leading to abdominal pain in susceptible individuals such as IBS patients.
Regarding intestinal bacteria and probiotics, various strains have been found to directly or indirectly influence intestinal barrier function (37). Probiotic supplementation, like Bifidobacteriaand Lactobacillae species, has shown promise in increasing the expression of TJ proteins and improving transepithelial resistance (38) (39). These probiotics can protect against acute colitis and revert inflammatory dysfunctions induced by cytokines such as TNF-alpha and IFN-gamma, thus improving barrier function in both humans and mice (40).Research into leaky gut treatments emphasizes the potential of functional foods and ingredients supporting an improvement of intestinal barrier function and reducing permeability.
Focus on the importance of finding innovative therapeutic strategies to prevent and revert intestinal barrier damage
Postbiotics are considered safe as they do not contain live bacteria, mitigating concerns associated with administering live microorganisms that might translocate across a damaged intestine (41) This innovative safety aspect offers a targeted approach to address LGS without the risks associated with live bacteria on fragile populations. Differently from probiotics that need to find the right environment to release beneficial metabolites, postbiotics provide an immediate and reparative action on both the impaired intestinal barrier and microbiota diversification (42) (43).
For instance, recent research has underscored the significant beneficial role of a specific bacterial strain, Akkermansia muciniphila (44), in reinforcing gut barrier (45) function across various conditions marked by heightened inflammation, such as obesity (46) (47), metabolic disorders (48) and autoimmune diseases (49). Researchers have later on demonstrated that this microorganism mainly exhorts its beneficial functions through the production and release of diverse metabolites, including proteins, short-chain fatty acids like acetate and propionate (50), but also through cell envelope components like phosphatidylethanolamine and peptidoglycans (47). These observations were further confirmed by a clinical study in obese patients reporting how daily administration of the pasteurized form of this microorganism markedly improves patients’ metabolic parameters, indicating its potential as a therapeutic intervention (46).
Hence, microorganism derived metabolites are identified as the true contributors to the beneficial effects associated with probiotics, and their ability to be administered at higher concentrations without the necessity for colonizing the gut provides significant flexibility in dosage and delivery. Further, as they derive from microbial fermentation, differently from prebiotics are devoid of side effects such as gas production. The selectivity of postbiotics can be tailored for specific applications, enhancing their efficacy in targeted treatment for disorders characterized by an impairment of the intestinal barrier integrity.

Experimental evidence about the protective and modulatory properties of postbiotics in different models of gut inflammation and intestinal barrier disruption
Effects of postbiotic in the treatment of Chron disease associated intestinal barrier damage in ex-vivo models
IBD is characterized by excessive inflammation in various parts of the intestinal wall, leading to mucosal damage, ulcerations, and fibrosis.
Probiotics have garnered attention for their potential therapeutic role in IBD, but caution is advised, as the efficacy of probiotics varies depending on the bacterial strain and clinical context. Clinical studies have highlighted potential risks associated with administering probiotics to patients with acute inflammation (51).
In our laboratory, we tested the immunomodulatory properties of three different probiotic strains, Lactobacillus paracasei B21060, Lactobacillus rhamnosus GG (LGG) and Lactobacillus plantarum NCIMB8826, on tissues derived from Chron patients using a polarized organ culture system(2).
In healthy tissues, inoculation with L. paracasei B21060 or L. rhamnosus GG (LGG)did not result in significant changes, with the cytokine secretion profile remaining similar to negative control samples. Surprisingly, stimulation with L. plantarum NCIMB8826 led to tissue deterioration, characterized by immune cell homing towards the epithelial layer, along with an upregulation of key inflammatory molecules such as CCL4 and Interleukin (IL)-1 beta. This suggests that in inflamed tissues with potentially increased intestinal permeability and bacterial translocation, otherwise harmless bacteria may exacerbate inflammation. By contrast, strains that were harmless on healthy tissues exacerbated the inflammation on IBD tissues. Therefore, caution is warranted when considering probiotic use in patients during the acute phase of IBD.
To overcome this challenge, Lactobacillus paracasei B21060 was used to produce postbiotics and the preparation was tested to explore its activity in ex-vivo organ culture after Salmonella thyphimurim (S. thyphimurium) infection of healthy tissues or on Crohn’s disease samples. Treatment with postbiotic resulted in reduction of TNF secretion paired by a reduction in ulceration of the epithelium and glandular destruction, histological features altered by S.thyphimurium infection.
When tested on tissue from CD patients, evaluation of NF-kB activation, a pathway associated with TNF production, showed reduced p65 translocation to the nucleus in samples treated with postbiotic, indicating a release of the inflammatory condition. Overall, these findings highlight the potential of postbiotics, (derived from L. paracasei B21060), to modulate inflammatory responses without compromising protecting anti-inflammatory mechanisms.
Effects of postbiotic in the prevention of Salmonella thyphimurium induced intestinal barrier disruption
To test the direct effect of postbiotics on IEB upon colonic epithelium injury induced by the pathogenic agent (S. thyphimurium) we performed both in vitro and in vivo studies(52).
Caco-2 cells were used to mimic the intestinal epithelial barrier in vitro and transepithelial electrical resistance (TEER) was used as an indicator of intestinal epithelial integrity. Treatment with postbiotic-derived from Lactobacillus paracasei CNCMI-5220 prevented S. thyphimurium barrier disruption, maintaining TEER values similar to those of non-infected cells.
Further analysis focused on TJs highlighted the ability of postbiotic-derived from L. paracasei CNCM I-5220 to protect TJ structures, altered by S. typhimurium infection. Importantly, the postbiotic did not exhibit direct antibacterial effects, but was able to restore the altered morphology of tight junctions after exposure to pathogen.
Intestinal barrier protective effect of postbiotic-derived from L. paracasei CNCMI-5220 was also evaluated in vivo. Mice treated orally for 10 days with the postbiotic showed reduced bacterial translocation to the colon and liver upon infection with S. typhimurium compared to controls. Moreover, the postbiotic preserved the integrity of the GVB and restored mRNA expression levels of α-Defensin and TGF-beta, markers of antimicrobial peptide release and anti-inflammatory response, respectively. Additionally, the postbiotic maintained gut microbiota composition preserving the abundance of the Ruminococcaceae family which was reduced upon S. typhimurium infection. These findings suggest that the postbiotic protects the intestinal barrier both in vitro and in vivo preventing pathogenic bacterial translocation.
Furthermore, the postbiotic was shown to directly interact with S. typhimurium, blocking biofilm formation and reducing bacterial pathogenicity. Additionally, biosurfactants isolated from the postbiotic, replicated the inhibitory effect on S. typhimurium biofilm formation exerted by the whole postbiotic, suggesting a direct role of these molecules in antibiofilm activity.
These findings provide evidence that postbiotics can have a protective role in preserving the integrity of IEB.
Conclusions and future perspectives
Dysregulation of the IEB has been linked to numerous intestinal and extra-intestinal disorders, characterized by increased intestinal permeability and chronic inflammation. These findings together suggest that postbiotics hold promise as a non-pharmacologic strategy for preventing and mitigating the leaky gut syndrome and its associated disorders, offering a novel approach to maintaining gut homeostasis and overall health.
Further studies are needed to dissect the full potential and molecular mechanisms at the base of protective effects of postbiotics on gut health.
Clinical studies should be performed to assess both the efficacy of postbiotics in the treatment of patients affected by LGS and as a possible combined therapeutic strategy to current therapies for pathologies characterized by mucosal barrier disruption extending their possible use in extra-intestinal disorders.



References and notes
- Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol19, 55–71 (2021).
- Tsilingiri, K. et al. Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut61, 1007–1015 (2012).
- Spadoni, I. et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science350, 830–4 (2015).
- Rooks, M. G. & Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat Rev Immunol16, 341 (2016).
- Castillo, D. J., Rifkin, R. F., Cowan, D. A. & Potgieter, M. The Healthy Human Blood Microbiome: Fact or Fiction? Front Cell Infect Microbiol9, (2019).
- Turner, J. R. Intestinal mucosal barrier function in health and disease. Nature Reviews Immunology 2009 9:119, 799–809 (2009).
- Camilleri, M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut68, 1516–1526 (2019).
- Ma, T. Y., Nighot, P. & Al-Sadi, R. Tight Junctions and the Intestinal Barrier. Physiology of the Gastrointestinal Tract 587–639 (2018) doi:10.1016/B978-0-12-809954-4.00025-6.
- Hartsock, A. & Nelson, W. J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochimica et Biophysica Acta (BBA) - Biomembranes1778, 660–669 (2008).
- Ménard, S., Cerf-Bensussan, N. & Heyman, M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol3, 247–259 (2010).
- Chakaroun, R. M., Massier, L. & Kovacs, P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients12, 1082 (2020).
- Spadoni, I. et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science350, 830–4 (2015).
- Groschwitz, K. R. & Hogan, S. P. Intestinal barrier function: Molecular regulation and disease pathogenesis. Journal of Allergy and Clinical Immunology124, 3–20 (2009).
- Ghosh, S. S., Wang, J., Yannie, P. J. & Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. 4, (2020).
- Odenwald, M. A. & Turner, J. R. The intestinal epithelial barrier: A therapeutic target? Nat Rev Gastroenterol Hepatol14, 9–21 (2017).
- Algieri, F. et al. Lactobacillus paracasei CNCM I-5220-derived postbiotic protects from the leaky-gut. Front Microbiol14, (2023).
- Citi, S. Intestinal barriers protect against disease. Science (1979)359, 1097–1098 (2018).
- Edelblum, K. L. & Turner, J. R. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol9, 715–720 (2009).
- Lee, B., Moon, K. M. & Kim, C. Y. Tight junction in the intestinal epithelium: Its association with diseases and regulation by phytochemicals. J Immunol Res2018, (2018).
- Fukui, H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm Intest Dis1, 135–145 (2016).
- Fasano, A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020 9:699, 69 (2020).
- Thomas, K. E., Sapone, A., Fasano, A. & Vogel, S. N. Gliadin Stimulation of Murine Macrophage Inflammatory Gene Expression and Intestinal Permeability Are MyD88-Dependent: Role of the Innate Immune Response in Celiac Disease. The Journal of Immunology176, 2512–2521 (2006).
- Teixeira, T. F. S., Collado, M. C., Ferreira, C. L. L. F., Bressan, J. & Peluzio, M. do C. G. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutrition Research32, 637–647 (2012).
- Cox, A. J. et al. Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab43, 163–166 (2017).
- Mouries, J. et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol (2019) doi:10.1016/j.jhep.2019.08.005.
- Sampson, T. R. et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell167, 1469-1480.e12 (2016).
- Carloni, S. et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science374, 439–448 (2021).
- Perez-Pardo, P. et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut68, 829–843 (2019).
- Clairembault, T., Leclair-Visonneau, L., Neunlist, M. & Derkinderen, P. Enteric glial cells: new players in Parkinson’s disease? Mov Disord30, 494–498 (2015).
- Lee, J. Y. et al. Molecular Pathophysiology of Epithelial Barrier Dysfunction in Inflammatory Bowel Diseases. Proteomes 2018, Vol. 6, Page 176, 17 (2018).
- Parekh, P. J., Balart, L. A. & Johnson, D. A. The Influence of the Gut Microbiome on Obesity, Metabolic Syndrome and Gastrointestinal Disease. Clin Transl Gastroenterol6, (2015).
- Pasini, E. et al. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC Heart Fail4, 220–227 (2016).
- Bischoff, S. C. et al. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol14, (2014).
- T, C. et al. Dietary Fibre-Based SCFA Mixtures Promote Both Protection and Repair of Intestinal Epithelial Barrier Function in a Caco-2 Cell Model. Food Funct8, (2017).
- Y, F., Y, W., P, W., Y, H. & F, W. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell Physiol Biochem49, (2018).
- VanHook, A. M. Butyrate benefits the intestinal barrier. Sci Signal8, ec135–ec135 (2015).
- Ohland, C. L. & MacNaughton, W. K. Probiotic bacteria and intestinal epithelial barrier function. American Journal of Physiology-Gastrointestinal and Liver Physiology298, G807–G819 (2010).
- J, K. et al. Regulation of Human Epithelial Tight Junction Proteins by Lactobacillus Plantarum in Vivo and Protective Effects on the Epithelial Barrier. Am J Physiol Gastrointest Liver Physiol298, (2010).
- Corridoni, D. et al. Probiotic Bacteria Regulate Intestinal Epithelial Permeability in Experimental Ileitis by a TNF-Dependent Mechanism. PLoS One7, e42067 (2012).
- S, R.-L. & KE, B. Probiotics and Commensals Reverse TNF-alpha- And IFN-gamma-induced Dysfunction in Human Intestinal Epithelial Cells. Gastroenterology130, (2006).
- Tsilingiri, K. & Rescigno, M. Postbiotics: what else? Benef Microbes4, 101–7 (2013).
- Zagato, E. et al. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nature Microbiology 2020 5:35, 511–524 (2020).
- Aguilar-Toalá, J. E. et al. Postbiotics — when simplification fails to clarify. Nature Reviews Gastroenterology & Hepatology 2021 18:1118, 825–826 (2021).
- Cani, P. D., Depommier, C., Derrien, M., Everard, A. & de Vos, W. M. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatol19, 625–637 (2022).
- Wells, J. M. et al. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol312, G171–G193 (2017).
- Depommier, C. et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med25, 1096–1103 (2019).
- Plovier, H. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med23, 107–113 (2017).
- Silvestri, A. et al. Biomimetic superabsorbent hydrogel acts as a gut protective dynamic exoskeleton improving metabolic parameters and expanding A. muciniphila. Cell Rep Med4, (2023).
- Fang, X., Li, F. jun & Hong, D. jun. Potential Role of Akkermansia muciniphila in Parkinson’s Disease and Other Neurological/Autoimmune Diseases. Curr Med Sci41, 1172–1177 (2021).
- Segers, A. & de Vos, W. M. Mode of action of Akkermansia muciniphila in the intestinal dialogue: role of extracellular proteins, metabolites and cell envelope components. Microbiome research reports2, (2023).
- Kothari, D., Patel, S. & Kim, S. K. Probiotic supplements might not be universally-effective and safe: A review. Biomedicine & Pharmacotherapy111, 537–547 (2019).
- Algieri, F. et al. Lactobacillus paracasei CNCM I-5220-derived postbiotic protects from the leaky-gut. Front Microbiol14, (2023).

