KEYWORDS
Microbiota
Prebiotics
Probiotics
Dysbiosis
Front-of-pack nutrition labelling
Post-biotics
Gut-skin axis
The human body hosts a number of different localized microbiotas beyond the gastro-intestinal one, recognized to play a significant role in maintaining host health, through different mechanisms. Today prebiotics, probiotics, and post-biotics are widely considered promising candidates able to modulate gut microbiota first and exert beneficial effects on other proximal body areas. This article summarizes their controversial uses in the life science industries, speculating on mechanisms of action, lack of regulation, and characterization profiles on one side, while highlighting extremely promising perspectives from both the market and scientific points of view.
Abstract
Introduction
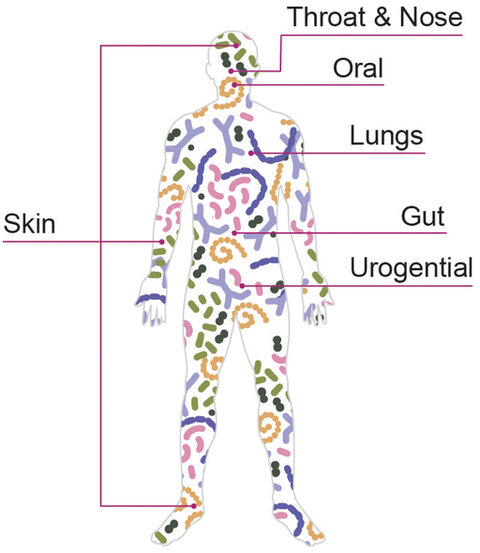
The human body hosts a number of different microbiomes, including those of the gut (stomach and intestines), uro-genital tract, oral cavity and skin, with different features depending on the different anatomic area.
Human microbiota presents different composition profile depending on the body areas, due to physical and chemical features of the local epithelium as well as exposition to external factors (dietary habits, environment, life style, drugs…).
The gut microbiota plays a significant role in maintaining host health, through the absorption or production of nutrients, regulation of energy and metabolic activity, modulation of immune response, and defence against pathogens.
Several studies have found that prebiotics, probiotics, and probiotic derivatives known as “post-biotic,” are key players able to exert beneficial effect to host such as improve intestinal microbiota homeostasis and inflammation, reduce the risk of obesity, type 2 diabetes and other cardiovascular diseases, regulate liver activity and central nervous system, by preventing against pathogen invasion, maintaining gut barrier integrity and modulating immune response and signalling pathways (1).
Prebiotics & synbiotics
In 2017 the definition of prebiotic has been reviewed by panel of experts from International Scientific Association for Probiotics and Prebiotics (ISAPP) in wider perspective: prebiotics have been described as “a substrate that is selectively utilized by host microorganisms conferring a health benefit”. This definition expands the concept of prebiotics (2):
- not only for oral intake, but direct administration to other microbially colonized body sites, such as the vaginal tract and skin;
- health benefits targeting gastrointestinal tract (inhibition of pathogens, immune stimulation, …), cardiometabolism (reduction in blood lipid levels, effects upon insulin resistance, …), mental health (metabolites that influence brain function, energy and cognition, …) among others;
- as definitive proof of causality is difficult to provide, human or animal studies are needed, showing a change in heath markers or symptoms after a specific influence on the microbial population;
- prebiotics are not only carbohydrate-based anymore, but other substances such as polyphenols and polyunsaturated fatty acids can be considered within the definition;
- the beneficial effect(s) on health must be confirmed in the target animal for its intended use and mediated through the microbiota.
The prebiotic selectiveness becomes the key point to exclusive synergies with certain group of bacteria instead of others, and is responsible for a direct effect on the microbiota composition and at clinical level, constituting the closest trait to the so-called “strain specificity” claimed for probiotics.
The first time the combination of probiotics and prebiotics was described as potential strategy for modulating the composition and/or function of the gut microbiota is dated back to thirty years ago. During the decades, the synbiotic concept has evolved, reaching the most recent definition according to ISAPP in 2019, where a distinction has been made between complementary and synergistic synbiotic. A complementary synbiotic consists of a probiotic combined with a prebiotic, designed to target resident microorganisms without co-dependent functions, while a synergistic synbiotic contains a substrate that is selectively utilized by co-administered microorganism(s) (3).
Probiotics
The most recent definition for probiotics as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” comes from the position statement proposed by ISAPP in 2018 (4). So far, the list of probiotics approved for food use includes three main categories: lactic acid-producing bacteria (Lactobacillus, Bifidobacterium, Enterococcus and more), yeast, and spore-forming Bacillus species.
Nowadays, current probiotic interventions are limited by some aspects that reduce their effectiveness and reputation to consumers. Firstly, probiotics are restricted to food-safe bacteria having Qualified Presumption of Safety (QPS) by the European Food Safety Authority (EFSA, 5) or Generally Recognised as Safe (GRAS) by the US Food and Drug Administration (FDA, 6). However, as most specie from the human gut microbiota are not considered food-safe a priori, such endogenous microbes are not permitted in human application, unless assessed safe under Novel Foods or New Dietary Ingredients legislations. This makes efficacy-driven innovation dependent from regulatory compliance and corresponding huge investments, as occurs with so-called “next generation probiotics” such as Akkermansia muciniphila, Faecalibacterium prausnitzii, Bacteroides fragilis, Clostridium butyricum and more. Secondly, when administering probiotics orally to target the gut microbiota, the main challenge is delivering an adequate number of viable cells after exposure to stomach acids and bile salts, thus allowing colonization, persistence and interaction at intestinal level. (7) Moreover the probiotic viability at the end of shelf-life should be guaranteed by reliable formulations and proper stability studies. Finally, harmonization of probiotics regulation should revise labelling rules and require to list not only species and viability at the end of shelf-life, but also the specific strain.
Post-biotics
Postbiotics, a newly categorized group of biotics, have been defined by the International Scientific Association of Probiotics and Prebiotics (ISAPP) in 2021 to replace prior inconsistent descriptions. The panel has defined postbiotics as "preparations of inanimate microorganisms and/or their components that confer health benefits on the host" (8).
However, this consensus statement proposes a common term to simultaneously indicate inactivated microorganisms as well as their specific by-products or components, putting “non-viable” bacteria, their fragments (complex matrices) or specific derivatives (molecular factors) on the same level in terms of characterization requisites, safety and efficacy (9). In literature, a different term has been previously adopted to identify dead but intact bacteria: “para-probiotics”, with the meaning of “beyond/aside from” probiotics, and used for inactivated -also reported as ghost –bacteria (10). Indeed, “heat-killed” or “tyndallized” terms simply inform about the inactivation method, while “non-viable” probiotics just refers to viability feature. Because of their intact structure, para-probiotics preserve their capacity to induce host pattern recognition receptors (such as TLRs) expression and activate innate and adaptive immunity, through inflammatory signalling pathways; the persisting adhesion proteins on cell membrane surfaces also allow them to adhere to different epithelia, mechanically preventing from the adhesion of pathogens, as the alive probiotics are used to do. (11).
On the other side, post-biotics may be defined as derivatives from microbial origin or crude cell extracts with complex chemical composition, and should denote specific pattern of molecules, whose profile complexity depends on their purification degree. Post-biotics should then include the complex mixture of metabolites secreted by probiotics such as cell-free supernatants, exopolysaccharides, enzymes, cell wall fragments, vitamins, teichoic acids, bacteriocins, peptides, aminoacids and proteins, short chain fatty acids (SCFA) and organic acid, bacterial lysates, bio-surfactants and exopolysaccharides (EPS) and other gut microbiota metabolites, with or without cell component (12).
Regardless the definition consensus and although post-biotics do not include live microorganisms, their added value is unanimous thanks to similar mechanisms characteristic of alive probiotics, without the potential risks associated with their intake: (i) no risk of bacterial translocation among vulnerable and immune-compromised subjects; (ii) no chances of acquisition and transfer of antibiotic resistance genes; (iii) easier standardization, transport, and storage; (iv) release of active molecules from the disrupted cells, stimulating epithelial cells more directly; (v) specific mechanisms of action by ligand-receptor interaction or biomolecular pathways (13).
On the other side, these three so different categories (inactivated cells, their fragments and metabolites) hardly match with a standardized and repeatable characterization profile, making a regulatory harmonization difficult and risking falling either in pharmaceutical legislation or Novel Food category, both requiring huge research and authorization investments. Moreover, stability at room temperature cannot be obvious as the shelf life of any food, supplement or drug is influenced by its components (inorganic, organic or enzymatic) that can inevitably undergo degradation processes (such as enzymatic modifications, Maillard reactions or oxidations), especially due to improper storage conditions and/ or not suitable packaging.
Interestingly, more recent research is turning towards potential application of post-biotics to other areas of the body, including the skin, vagina and oral cavity (7). Indeed, postbiotic properties open possibilities to generate them from a considerably more diverse range of microbes, including microbial taxa that lack a history of safety and are therefore excluded as probiotics.
Emerging markets for biotics
Likewise, the correlation between gut dysbiosis and related distress has raised the need for products that address the microbiota unbalances at their root. Nowadays both the scientific community and the skin care industry are increasingly aware about the link between a healthy skin and the status of its microbiota. Despite the use of oral probiotics for skin infections or atopic conditions in literature is dated back to over 10 years, the skin targets are opening to topics such as anti-ageing, sensitiveness, prevention from pollution, sun tanning, moisturizers, oily skin, body odours, up to scalp/ hair care and so on… The breaking point with the past is the growing consciousness of consumers, regardless the mode of use, thus no matters if it is oral intake for skin supplements or topical application for cosmetics or medical devices. From a market perspective however, it is much easier to ride this wave for cosmetics because of the lack in regulation and the easy access to the target organ – the skin – for testing clinical efficacy.
One big challenge providing different development speeds between cosmetic and nutraceutical markets is the regulation gap. Indeed, supplements have strict limitations on health claims, require medical advertising for promotion, need to comply with labelling requirements such as viability at the end of shelf-life and strain code indication in some countries. On the other side, cosmetics are suspended in a grey zone characterized by few and generic INCI names (Lactobacillus, ferment) that do not help to discriminate among inactivated bacteria, lysate or isolated metabolites and especially to distinguish between one strain or another. Also, beauty claims are easier than health claims due to a less strict Regulation based on positive and negative lists, but lacking formal approval process by the authority, while the control only comes when the product is on the market by consumer associations or advertising standard alliance.
The skin microbiome and the gut-skin axis
Likewise, the correlation between gut dysbiosis and related distress has raised the need for products that address the microbiota unbalances at their root. Nowadays both the scientific community and the skin care industry are increasingly aware about the link between a healthy skin and the status of its microbiota. Despite the use of oral probiotics for skin infections or atopic conditions in literature is dated back to over 10 years, the skin targets are opening to topics such as anti-ageing, sensitiveness, prevention from pollution, sun tanning, moisturizers, oily skin, body odours, up to scalp/ hair care and so on… The breaking point with the past is the growing consciousness of consumers, regardless the mode of use, thus no matters if it is oral intake for skin supplements or topical application for cosmetics or medical devices. From a market perspective however, it is much easier to ride this wave for cosmetics because of the lack in regulation and the easy access to the target organ – the skin – for testing clinical efficacy.
One big challenge providing different development speeds between cosmetic and nutraceutical markets is the regulation gap. Indeed, supplements have strict limitations on health claims, require medical advertising for promotion, need to comply with labelling requirements such as viability at the end of shelf-life and strain code indication in some countries. On the other side, cosmetics are suspended in a grey zone characterized by few and generic INCI names (Lactobacillus, ferment) that do not help to discriminate among inactivated bacteria, lysate or isolated metabolites and especially to distinguish between one strain or another. Also, beauty claims are easier than health claims due to a less strict Regulation based on positive and negative lists, but lacking formal approval process by the authority, while the control only comes when the product is on the market by consumer associations or advertising standard alliance.
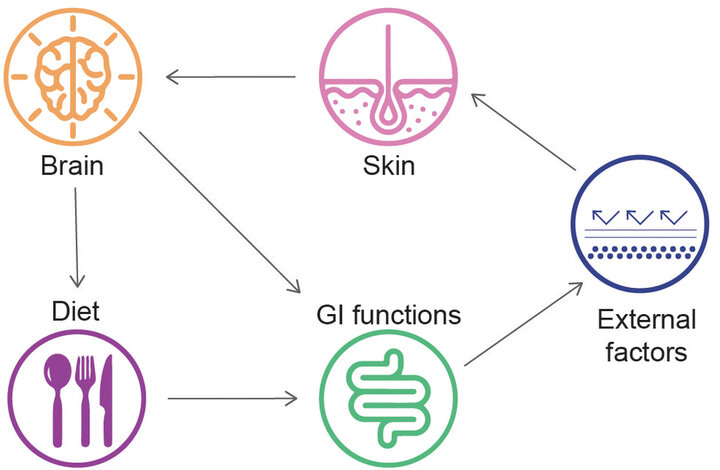
Psychological distress & unbalanced diet could lead to gut microbiota alteration. An intestinal dysbiotic status can increase gut permeability and endotoxin translocation, thus triggering skin discomforts.
Mechanisms involved are yet to be properly identified but in general, the improvement of intestinal permeability and the immune crosstalk among cells are the main drivers to reduce inflammation and shift bacterial diversity from a pathogenic profile to a balanced microbiota. However, specific mechanisms and effectors responsible for skin impairments like gene expression, activation of surface proteins, endotoxins translocations, and release of functional metabolites are still relatively underdeveloped so far. A further complication in the gut-skin axis relies in the brain component entering the game, where neurotransmitters are cause and consequences of skin impairments. Psychological distress and emotional state alone or in combination with high fat diet or processed foods devoid of fibers, cause alterations to gastro-intestinal function and microbiota profile. Loss of normal microbial biofilm and poor biodiversity cause intestinal permeability and endotoxins systemic translocations, increasing inflammation and oxidative stress. In those genetically susceptible subjects to skin diseases and symptoms, this cascade exacerbates these conditions and adds additional psychosomatic impairment affecting mood, cognition and sleep in a vicious cycle (14).
References and notes
- Liu Y, Wang J, Wu C. Modulation of Gut Microbiota and Immune System by Probiotics, Pre-biotics, and Post-biotics. Front Nutr. 2022 Jan 3;8:634897. doi: 10.3389/fnut.2021.634897. PMID: 35047537; PMCID: PMC8761849.
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017 Aug;14(8):491-502. doi: 10.1038/nrgastro.2017.75. Epub 2017 Jun 14. PMID: 28611480.
- Yadav MK, Kumari I, Singh B, Sharma KK, Tiwari SK. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl Microbiol Biotechnol. 2022 Jan;106(2):505-521. doi: 10.1007/s00253-021-11646-8. Epub 2022 Jan 11. PMID: 35015145; PMCID: PMC8749913.
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014 Aug;11(8):506-14. doi: 10.1038/nrgastro.2014.66. Epub 2014 Jun 10. PMID: 24912386.
- https://www.efsa.europa.eu/en/topics/topic/qualified-presumption-safety-qps#elenco-dei-microrganismi-con-stato-qps-elenco-qps-ed-elenco-delle-notifiche
- https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras
- Scott E, De Paepe K, Van de Wiele T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules. 2022 Nov 4;12(11):1640. doi: 10.3390/biom12111640. PMID: 36358990; PMCID: PMC9688025.
- The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021 Sep;18(9):671. doi: 10.1038/s41575-021-00481-x. Erratum for: Nat Rev Gastroenterol Hepatol. 2021 Sep;18(9):649-667. Erratum in: Nat Rev Gastroenterol Hepatol. 2021 Jul 29; PMID: 34131318; PMCID: PMC8387228.
- Aguilar-Toalá JE, Arioli S, Behare P, Belzer C, Berni Canani R, Chatel JM, D'Auria E, de Freitas MQ, Elinav E, Esmerino EA, García HS, da Cruz AG, González-Córdova AF, Guglielmetti S, de Toledo Guimarães J, Hernández-Mendoza A, Langella P, Liceaga AM, Magnani M, Martin R, Mohamad Lal MT, Mora D, Moradi M, Morelli L, Mosca F, Nazzaro F, Pimentel TC, Ran C, Ranadheera CS, Rescigno M, Salas A, Sant'Ana AS, Sivieri K, Sokol H, Taverniti V, Vallejo-Cordoba B, Zelenka J, Zhou Z. Postbiotics - when simplification fails to clarify. Nat Rev Gastroenterol Hepatol. 2021 Nov;18(11):825-826. doi: 10.1038/s41575-021-00521-6. PMID: 34556825.
- Taverniti V, Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr. 2011 Aug;6(3):261-74. doi: 10.1007/s12263-011-0218-x. Epub 2011 Apr 16. PMID: 21499799; PMCID: PMC3145061.
- Teame T, Wang A, Xie M, Zhang Z, Yang Y, Ding Q, Gao C, Olsen RE, Ran C, Zhou Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front Nutr. 2020 Oct 22;7:570344. doi: 10.3389/fnut.2020.570344. PMID: 33195367; PMCID: PMC7642493.
- Scarpellini E, Rinninella E, Basilico M, Colomier E, Rasetti C, Larussa T, Santori P, Abenavoli L. From Pre- and Probiotics to Post-Biotics: A Narrative Review. Int J Environ Res Public Health. 2021 Dec 21;19(1):37. doi: 10.3390/ijerph19010037. PMID: 35010297; PMCID: PMC8750841.
- Siciliano RA, Reale A, Mazzeo MF, Morandi S, Silvetti T, Brasca M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients. 2021 Apr 8;13(4):1225. doi: 10.3390/nu13041225. PMID: 33917707; PMCID: PMC8068161.
- Bowe WP, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis - back to the future? Gut Pathog. 2011 Jan 31;3(1):1. doi: 10.1186/1757-4749-3-1. PMID: 21281494; PMCID: PMC3038963.

