Beauty from within
Efficacy of oral curcumin extract microcapsules on skin appearance
Teresa Negra1* , Alan Connolly, PhD2 , M.Carmen Lidón-Moya3, Gemma Mola4, Raquel Delgado5
*Corresponding author
1. Technical NBD Manager, Lubrizol Nutraceuticals, Barcelona, Spain
2. Global R&D Manager, Lubrizol Nutraceuticals, Barcelona, Spain
3. R&D Project Leader-Scientist, Lipotec S.A.U., Barcelona, Spain
4. Global Claim Substantiation Manager, Lipotec S.A.U., Barcelona, Spain
5. Global Senior Technical Director, Lubrizol, Barcelona, Spain



KEYWORDS
Turmeric extract
Curcumin
Microencapsulation
Skin
Antioxidant
Nutricosmetic
Abstract
Curcumin, the main active component in turmeric, is a powerful natural antioxidant whose benefits can be limited due to low bioavailability. We report a clinical study with 63 healthy women assessing the efficacy of a microencapsulated natural curcumin extract for improving facial skin appearance. Skin analysis highlighted that a daily intake of 2g of the microencapsulated curcumin for 42 days significantly decreases wrinkle area compared to T0, reduced skin redness and brown spots compared to T0 and placebo, and improved skin luminosity, homogeneity, and skin oxidative stress. In conclusion, the microencapsulated curcumin under study can be considered an effective nutricosmetic ingredient as it reduces skin oxidation, improves skin appearance, and helps mitigate signs of ageing.
Introduction
Skin is the largest organ of the human body and acts as a barrier between internal and external environments. Skin possesses a rich vascular network which is involved in feeding tissues, thermoregulation, wound healing, immune reactions, and blood pressure control (1). Exposure to external factors can induce skin ageing, distinguishing it from the aging processes of other tissues, and potentially leading to premature manifestations. Skin quality deteriorates due to the synergistic effects of chronological ageing, photo-ageing, hormonal deficiencies, and environmental factors. Skin ageing induces distinct structural changes that impact its youthful appearance and physiological functions, including permeability, lipid and sweat production, pigmentation, sallowness, laxity, wrinkling, angiogenesis, immune function, vitamin D synthesis, wound healing, and atrophy. These changes also increase vulnerability to external stimuli and the development of various diseases (2, 3).
The main cause of ageing is the lifelong accumulation of molecular damage mainly due to reactive oxygen species (ROS). External factors such as tobacco, chemicals, pollution and especially UV radiation increase ROS, leading to the cutaneous accumulation of advanced glycation end products (AGEs). This accumulation triggers inflammatory processes that activate cytokines, affecting DNA, RNA and proteins, downregulating collagen synthesis, ultimately resulting in thinner and more fragile skin. Oxidative stress also triggers changes in skin pigmentation, leading to the appearance of age-related spots (3, 4, 5, 6).
Topical treatments are commonly used to prevent ageing and protect the skin, but our diet can also play a crucial role in promoting skin health. Common skin conditions like acne, atopic dermatitis, ageing, and photoprotection are significantly affected by our diet. Essential nutrients benefit the structural and functional integrity of damaged skin and diets with high antioxidant components, such as vitamin C or polyphenols, promote skin health, minimizing the harmful effects of UV exposure. A balanced diet, combined with cosmetics and oral supplements, could represent a comprehensive approach to improving skin health, reducing ageing, and enhancing reactive skin (7, 8, 9, 10, 11).
Turmeric, a widely used spice derived from the rhizomes of Curcuma longa,is composed of active ingredients called curcuminoids, including curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Curcumin, the most abundant of these curcuminoids, is typically extracted from turmeric to enrich its concentration. Curcumin is a powerful antioxidant, anti-inflammatory agent and efficient NfkB inhibitor, which suggests it could also be effective as a natural aid to delay ageing (12, 13). It reduces ROS and AGE formation while also eliminating AGE-induced disturbances through various mechanisms (14, 15, 16). By reducing ROS, curcumin modulates collagen synthesis, which is demonstrated by the improvement in wound healing it exerts (17). Several studies have concluded that turmeric/curcumin-based products provide therapeutic benefits for skin health. (19) However, the preventive and therapeutic properties of curcumin can be limited due to its low solubility and, as a result, limited bioavailability.
Microencapsulation techniques can enhance the solubility and dispersibility of poorly soluble active ingredients. This technique leads to increased bioavailability of curcumin, thereby enhancing its absorption and enabling its use as an active ingredient in skincare formulations (18, 19). Curcumin is considered safe, with an acceptable daily intake of 3 mg/kg body weight as set by the European Food Safety Authority (EFSA) (20). Curcumin alone is considered as a non-authorized novel food. However, when the aforementioned curcuminoids are present in the same proportion as in the Curcuma longa root, they are not considered as novel food in food supplements. Other similar compounds or processes need individual assessment for regulatory status (21).
In this clinical study, we evaluated the benefits of the oral intake of a microencapsulated Curcuma longa extract composed of curcumin, demethoxycurcumin, and bisdemethoxycurcumin (with curcumin being the most abundant) nutraceutical ingredient (CCmc) produced using patented technology on the skin of healthy women (22). This microencapsulation process enhances the dispersibility of curcuminoids in water and increases their absorption within the body. This enhanced bioavailability was previously demonstrated through a clinical trial involving 10 participants, where blood serum curcumin levels were analyzed and compared over an 8-hour period following ingestion of curcumin in the form of CCmc and unencapsulated curcumin under fasting conditions. CCmc consistently exhibited higher blood curcumin levels at all analyzed time points following oral intake, with the highest levels observed 1 hour after ingestion. The results indicated that CCmc increased curcumin levels at different time points after the intake, whereas the nonencapsulated curcumin extract did not show any significant increase at any of the tested time points (23).
Prior to these clinical studies, several in vitro studies were conducted with human epidermal cells to evaluate the antioxidant capacity of CCmc. A comparative study of ROS levels versus a green tea extract on human dermal fibroblasts showed that CCmc has superior antioxidant power while another study quantifying AGEs on human keratinocytes exposed to UV radiation demonstrated a significant protective effect of CCmc against UV-induced glycation (23).

Materials and methods
Monocentric double-blind placebo control clinical study
The product under study was CurcushineTM microcapsules (CCmc), a trademark of Lubrizol Advanced Materials, Inc or its affiliates. CCmc is a microencapsulated natural curcuminoid extract obtained from Curcuma longa. CCmc is enriched in the three curcuminoids; curcumin, demethoxycurcumin, and bisdemethoxycurcumin (with curcumin being the most abundant). For the study, 63 healthy women aged 18 to 50 years with facial skin imperfections (dark spots and/or acne masks) and/or fine lines in the crow's feet area were recruited. To avoid major hormonal changes during the study, women who were pregnant, planning to conceive, breastfeeding, or recently stopped breastfeeding, as well as those with menopausal or premenopausal symptoms, were excluded. The panel ultimately consisted of 57 women aged between 21 to 50 years old.
Volunteers were divided into two groups. One group (n=27) took an active food supplement sachet daily (20g of water-soluble powder with 2g of CCmc, providing 0.5g of curcuminoids), and the other group (n=30) took a placebo supplement sachet daily (20g of water-soluble powder). Neither the study personnel nor the subjects were aware of the product names and compositions. Supplements were assigned according to a randomization list.
Fifteen days before study and during the treatment period, all volunteers used the same placebo cream (whole face, twice a day), and a cleanser (only at night, before placebo cream). The use of any other topical treatments was not allowed.
The study lasted 42 days, with measurements taken before and after 42 days (T42d) of product use. Evaluations were conducted in a room with controlled temperature (22±2ºC) and humidity (50±10% RH), with a 20-minute acclimatization period of the test area.
Clinical study testing methods
3D microtopography
To evaluate wrinkle parameters, real 3D microtopography images of the crow’s feet and forehead areas were taken with the PRIMOS lite system (Canfield), a three-dimensional measurement device. To ensure reproducibility of conditions throughout the study, measurements were carried out using PRIMOS face device (Canfield) and volunteers wore a black mobcap. Variations of wrinkle area parameter were determined by evaluating one randomized region (left or right) with the PRIMOS software (Canfield).
Macrophotographs
Lateral and frontal macrophotographs of the face were taken with the CameraScan software (Orion Concept) under standardized lighting conditions in a dark room using a high-resolution Nikon D7000 camera. To ensure reproducibility the volunteers wore a black mobcap and were positioned on a bench (HeadScan V04 Face, Orion Concept). Images were taken in parallel polarized light (lens filter at 0º to flash filter) and cross polarized light (lens filter at 90º to flash filter). To determine variations of L*, a* and b* parameters, frontal macrophotographs were evaluated using CieLab software. A higher L* indicated increased skin luminosity and radiance. A lower a* indicated less redness spots and erythema, while a lower in b* indicated fewer brown spots. To visualize decrease in brown spots, a facial mapping was done by overlaying pixels corresponding to the maximum CIEb* values on the original image of each volunteer. To determine variations in skin homogeneity, a region in the cheek area was evaluated from lateral macrophotographs using Framescan software (Orion), with a pigmentation filter. A higher homogeneity parameter indicates less heterogeneous skin with fewer imperfections, such as brown spots.
Skin antioxidant status
D-squame stripping discs were used to sample the cheek. Each disc was analyzed by chromatography to determine MDA (Malondialdehyde) levels, a marker of oxidative stress from lipid peroxidation. Reduced MDA levels indicated improved skin antioxidant status.
Clinical study statistical analysis
A descriptive statistical analysis of the results was performed including the calculation of mean values, standard error of the mean (SEM) and mean variation (%) calculated as:

Normality test (Shapiro-Wilk test) was performed to assure normal distribution of the data obtained. If normal distribution of the data was verified, t-Student test was applied to compare the values obtained before application versus each time-point evaluated. For non-normal distributions, non-parametric test (Wilcoxon test) was used.
Results
The results in Figure 1A indicate a significant decrease of wrinkle area (mm2) in the crow’s feet region of 10.32% (p=0.0195) after 42 days of supplementation with CCmc. Moreover, a visual reduction in the appearance of wrinkles could also be observed in the facial images (Figure 1B).
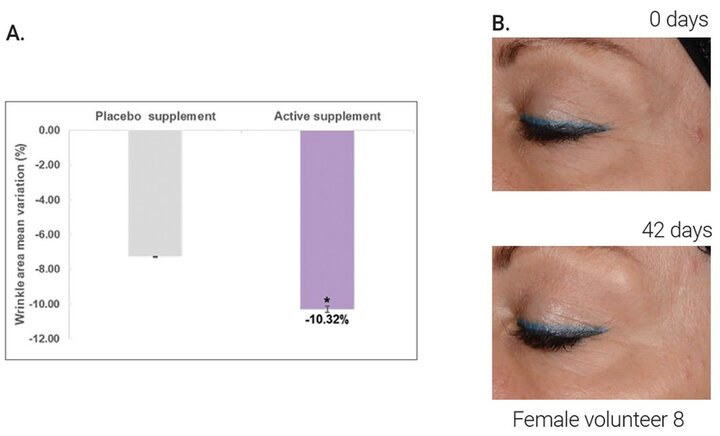
Figure 1. A) Variation (%) of the wrinkle area (mm2) in the crow´s feet region obtained after 42 days taking active supplement (*p<0.05 vs. initial time, calculated using a paired Student´s t-test after checking the normality distribution by a Shapiro-Wilk test). B) Representative images showing the anti-wrinkle effect after 42 days of supplementation with CCmc.
A reduction of the wrinkle volume (mm3) was also observed in the forehead area with an average decrease of 5.14% (p=0.2605) was obtained after 42 days of taking the active supplement sachet (Figure 2A) with respect to initial time. As demonstrated in Figure 2B, the L* parameter for facial skin showed a 0.62% (p=0.6246) improvement after 42 days of supplementation with CCmc compared to the initial measurement, indicating that the volunteers' skin became more radiant. On the other hand, skin redness was also evaluated. The results showed a decrease of a* parameter in a statistically significant manner by 1.95% (p=0.0007) with respect to initial time (Figure 2C) after 42 days of supplementation with CCmc.
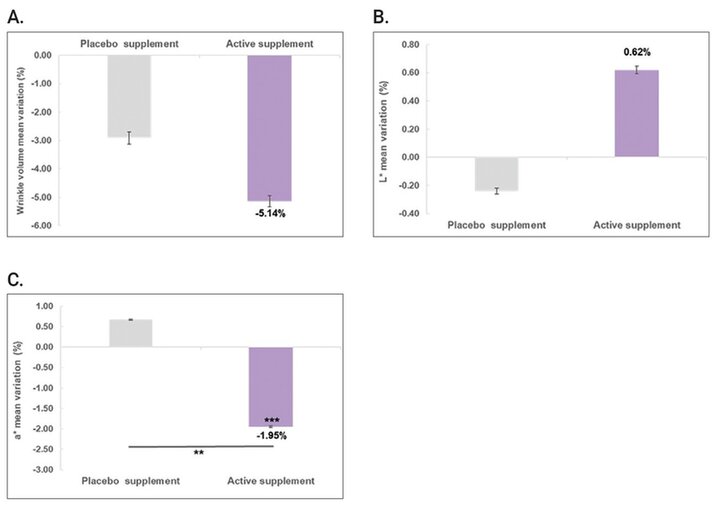
Figure 2. A) Variation (%) of the wrinkle volume in the forehead area. B) Variation (%) of L* parameter in the skin face. C) Variation (%) in the a* parameter in the skin face obtained after 42 days of supplementation with CCmc (vs. initial time: ***p<0.001; vs. placebo: **p<0.01, calculated using a Student’s t-test after checking the normality distributions by a Shapiro-Wilk test).
Brown spots were also evaluated. As shown in Figure 3A, the b* parameter decreased by 3.77% (p=0.0001) in a statistically significant manner after 42 days of supplementation with CCmc compared to initial time. A reduction in brown spots could also be visualized in the facial images (Figure 3B). Subsequently, variations in skin color homogeneity were also assessed. The results in Figure 3C indicated an average increase of 5.48% (p=0.1605) in skin color homogeneity after 42 days of supplementation with CCmc, compared to the initial measurement. Finally, the evaluation of skin antioxidant status revealed a statistically significant reduction of 7.35% (p=0.1022) in MDA levels after 42 days of supplementation with CCmc (Figure 3D). This decrease indicated an improvement in the skin´s antioxidant status.
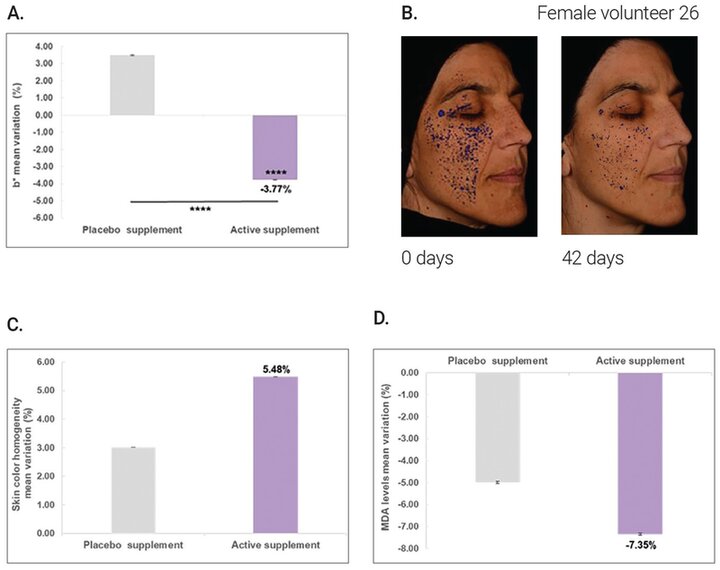
Figure 3. A) Variation (%) in the b* parameter in the skin face obtained after 42 of supplementation with CCmc (vs. initial time: ****p<0.0001; vs. placebo: ****p<0.0001, calculated using Student’s t-test after checking the normality distributions by a Shapiro-Wilk test). B) Representative images a reduction in brown spots after 42 days of treatment. A facial mapping of the pixels corresponding to the maximum values of b* parameter was superimposed on the original image of the volunteer. C) Variation (%) of skin color homogeneity on the cheek. D) Variation (%) of MDA levels on the cheek area obtained after 42 days of supplementation with CCmc.
Discussion
This clinical study focused on the impact of supplementation with a microencapsulated curcumin extract on signs of ageing and the appearance of facial epidermis in healthy women. Most of available studies evaluated the benefits of curcumin for skin conditions such as acne or atopic skin, either topically or ingestible, rather than on the appearance of healthy facial skin. This study highlights how epidermal ageing can be reduced by supplementation with a bioavailable source of natural curcuminoids. Curcumin, the most abundant of the curcuminoids present in the CCmc, has antioxidant, anti-inflammatory and antimicrobial properties and its beneficial effects have been demonstrated both for the treatment of skin diseases and for slowing tissue degeneration (12, 13). Incorporating it into the diet through supplementation can favor the modulation of skin health and beauty; however, its low solubility and intestinal absorption limit its potential benefits. The patented microencapsulation technology applied to CCmc naturally enhances the absorption of curcumin without altering the active ingredient’s structure. This study shows that, after oral ingestion of the CCmc, the microencapsulated curcuminoids are effectively released and absorbed within the body, allowing them to exert a positive effect on the skin.
The design of this study enabled us to assess the ability of CCmc to improve and delay the signs of ageing on facial epidermis. Wrinkles as well as pigmentation, homogeneity and luminosity of the skin were evaluated. After 42 days of supplementation, all the parameters evaluated showed improvement. A significant effect on reducing the area of wrinkles on crow's feet was observed as well as decrease in the volume of forehead wrinkles. Skin luminosity increased, with a significant decrease in skin redness and brown spots, leading to improved skin color homogeneity. Finally, the analysis of MDA levels, a biomarker of oxidative stress, showed a reduction in its levels compared to placebo. This indicates that CCmc microcapsules reduce oxidative stress in the epidermis layer, which may lead to a protective effect on its proteins and structures that ultimately translate into noticeable improvements in the appearance of the skin.
Conclusion
This clinical study demonstrates the skin benefits of CCmc, a nutraceutical ingredient based on a natural curcumin extract microencapsulated using patented technology. This technology imparts high water dispersible properties to the curcumin and other curcuminoids, enhancing their absorption by the body and leading to visible benefits in the epidermal layer of the skin. The study shows a significant decrease in wrinkle area, reduction of redness and brown spots, and a clear trend towards improved skin luminosity, homogeneity, and reduced oxidative stress. This positions CCmc as an efficient nutricosmetic ingredient capable of improving skin appearance and mitigating signs of ageing. The observed benefits of CCmc are likely due to the reduction of ROS and modulation of collagen synthesis. Therefore, CurcushineTM microcapsules can be considered a beneficial nutraceutical ingredient that improves skin when included in a standard diet. However, further studies are required to validate these findings.
References and notes
- Lopez-Ojeda W, Pandey A, Alhajj M, Oakley AM. Anatomy, Skin (Integument). 2022 Oct 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 28723009. https://pubmed.ncbi.nlm.nih.gov/28723009/
- Chaudhary M, Khan A, Gupta M. Skin Ageing: Pathophysiology and Current Market Treatment Approaches. Curr Aging Sci. 2020;13(1):22-30. doi: 10.2174/1567205016666190809161115. PMID: 31530270; PMCID: PMC7403684. https://pubmed.ncbi.nlm.nih.gov/31530270/
- Gkogkolou P, Böhm M. Advanced glycation end products: Key players in skin aging? Dermatoendocrinol. 2012 Jul 1;4(3):259-70. doi: 10.4161/derm.22028. PMID: 23467327; PMCID: PMC3583887. https://pubmed.ncbi.nlm.nih.gov/23467327/
- Benedetto AV. The environment and skin aging. Clin Dermatol. 1998 Jan-Feb;16(1):129-39. doi: 10.1016/s0738-081x(97)00193-4. PMID: 9472443. https://pubmed.ncbi.nlm.nih.gov/9472443/
- Rittié L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002 Sep;1(4):705-20. doi: 10.1016/s1568-1637(02)00024-7. PMID: 12208239. https://pubmed.ncbi.nlm.nih.gov/12208239/
- Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015 May;21:16-29. doi: 10.1016/j.arr.2015.01.001. Epub 2015 Jan 31. PMID: 25653189. https://pubmed.ncbi.nlm.nih.gov/25653189/
- Cao C, Xiao Z, Wu Y, Ge C. Diet and Skin Aging-From the Perspective of Food Nutrition. Nutrients. 2020 Mar 24;12(3):870. doi: 10.3390/nu12030870. PMID: 32213934; PMCID: PMC7146365. https://pubmed.ncbi.nlm.nih.gov/32213934/
- Schagen SK, Zampeli VA, Makrantonaki E, Zouboulis CC. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012 Jul 1;4(3):298-307. doi: 10.4161/derm.22876. PMID: 23467449; PMCID: PMC3583891. https://pubmed.ncbi.nlm.nih.gov/23467449/
- Mukherjee PK, Maity N, Nema NK, Sarkar BK. Bioactive compounds from natural resources against skin aging. Phytomedicine. 2011 Dec 15;19(1):64-73. doi: 10.1016/j.phymed.2011.10.003. Epub 2011 Nov 23. PMID: 22115797. https://pubmed.ncbi.nlm.nih.gov/22115797/
- Sun M, ‘et al.’. Effects of Natural Polyphenols on Skin and Hair Health: A Review. Molecules. 2022 Nov 14;27(22):7832. doi: 10.3390/molecules27227832. PMID: 36431932; PMCID: PMC9695112. https://www.mdpi.com/1420-3049/27/22/7832
- Piccardi N, Manissier P. Nutrition and nutritional supplementation: Impact on skin health and beauty. Dermatoendocrinol. 2009 Sep;1(5):271-4. doi: 10.4161/derm.1.5.9706. PMID: 20808515; PMCID: PMC2836433. https://pubmed.ncbi.nlm.nih.gov/20808515/
- Sikora E, Bielak-Zmijewska A, Mosieniak G, Piwocka K. The promise of slow down ageing may come from curcumin. Curr Pharm Des. 2010;16(7):884-92. doi: 10.2174/138161210790883507. PMID: 20388102. https://pubmed.ncbi.nlm.nih.gov/20388102/
- Tang Y, Chen A. Curcumin eliminates the effect of advanced glycation end-products (AGEs) on the divergent regulation of gene expression of receptors of AGEs by interrupting leptin signaling. Lab Invest. 2014 May;94(5):503-16. doi: 10.1038/labinvest.2014.42. Epub 2014 Mar 10. PMID: 24614199; PMCID: PMC4006284. https://pubmed.ncbi.nlm.nih.gov/24614199/
- Barzegar A, Moosavi-Movahedi AA. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS One. 2011;6(10):e26012. doi: 10.1371/journal.pone.0026012. Epub 2011 Oct 10. PMID: 22016801; PMCID: PMC3189944. https://pubmed.ncbi.nlm.nih.gov/22016801/
- Vaughn AR, Branum A, Sivamani RK. Effects of Turmeric (Curcuma longa) on Skin Health: A Systematic Review of the Clinical Evidence. Phytother Res. 2016 Aug;30(8):1243-64. doi: 10.1002/ptr.5640. Epub 2016 May 23. PMID: 27213821. https://pubmed.ncbi.nlm.nih.gov/27213821/
- Alizadeh M, Kheirouri S. Curcumin against advanced glycation end products (AGEs) and AGEs-induced detrimental agents. Crit Rev Food Sci Nutr. 2019;59(7):1169-1177. doi: 10.1080/10408398.2017.1396200. Epub 2017 Nov 29. PMID: 29185795. https://pubmed.ncbi.nlm.nih.gov/29185795/
- Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006 Oct;290(1-2):87-96. doi: 10.1007/s11010-006-9170-2. Epub 2006 Jun 13. PMID: 16770527. https://pubmed.ncbi.nlm.nih.gov/16770527/
- Kasprzak-Drozd K, ‘et al.’. Potential of Curcumin in the Management of Skin Diseases. Int J Mol Sci. 2024 Mar 23;25(7):3617. doi: 10.3390/ijms25073617. PMID: 38612433; PMCID: PMC11012053. https://pubmed.ncbi.nlm.nih.gov/38612433/
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007 Nov-Dec;4(6):807-18. doi: 10.1021/mp700113r. Epub 2007 Nov 14. PMID: 17999464. https://pubmed.ncbi.nlm.nih.gov/17999464/
- European Food Safety Authority. (2014). Refined exposure assessment for curcumin (E 100). EFSA Journal, 12(10), 3876. https://doi.org/10.2903/j.efsa.2014.3876
- European Commission. (2022). Commission Implementing Regulation (EU) 2022/961 of 20 June 2022 authorising the placing on the market of tetrahydrocurcuminoids as a novel food and amending Implementing Regulation (EU) 2017/2470 (Text with EEA relevance). Official Journal of the European Union, L 165, 41-45. https://eur-lex.europa.eu/eli/reg_impl/2022/961/oj
- Zanoni F., Zoccatelli G, Vakarelova M, Chignola R. Multi-layered particles. 2020 IP number WO2020104970A2. https://patents.google.com/patent/WO2020104970A2/en
- Curcushine Brochure. 2024 https://www.lubrizol.com/-/media/Lubrizol/Health/Documents/Nutraceuticals/CURCUSHINE-microcapsules-brochure.pdf

