What is the objective of the ILSI Europe Probiotics Task Force and why is it relevant to the industry?
The Task Force aims to advance the understanding of probiotics and their health benefits in the broadest sense. We do this by exchanging ideas with scientists from industry and academia, and when possible, with regulatory bodies. Together, we identify scientific questions, then we commission a group of experts to address them. The research is precompetitive and the presence of at least 50% of academic scientists ensures a balanced outcome. We share the findings with the scientific community in industry, academia and public sector, and provide information for interested laypersons. We believe that honest communication about probiotics benefits the whole field, including industry. ILSI Europe members, of course, have the advantage that they can suggest topics they’d like to see addressed.
What are some of the key challenges faced by the probiotics industry today, and how does the Task Force aim to address them?
A continued challenge for the probiotic industry is the health claim regulation in Europe. But that’s a regulatory challenge. What we have are scientific opportunities. Technology is advancing and we can investigate things we could not even dream of 10 or 15 years ago. The task force members brainstorm on such opportunities to advance the field. When a topic is identified, members draft a research proposal. Once approved, the work is carried out by an expert group who regularly report back to the task force. ILSI Europe’s reputation and academic network enable the inclusion of top scientists in the expert group. That is a great opportunity.
What strategies does the Task Force use to communicate its findings to a broader audience, including consumers and policymakers?
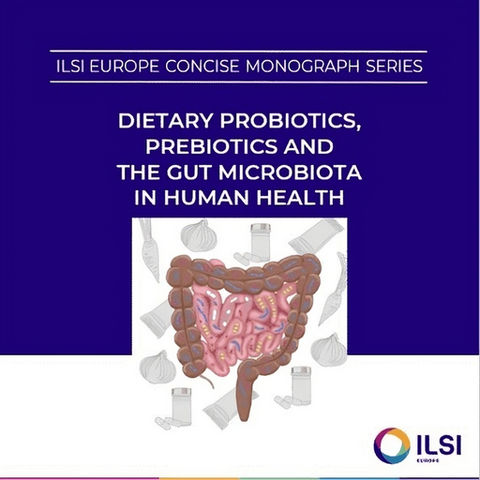
We have published a concise monograph on probiotic, prebiotics and the gut microbiota. It explains in layperson terms the principles of biotics and what the intestinal microbiota means for our health. That monograph is available in seven languages! For the more ‘hard-core’ science we do, we make sure the articles are open access, thus available to everyone. We also connect with news outlets to disseminate what we have done.
As an expert in the industry, what has been a highlight of the Task Force’s work for you?
I can think of two. One is the concise monograph I mentioned above. The other is our paper about the difference in physiology and ecology of the small intestine compared to the large intestine. We casually tend to talk about ‘intestinal microbiota’, but that usually refers only to the faecal microbiota, which may –or may not– be representative for the colon. We have very little knowledge about what happens in the small intestine. For probiotics the paper highlights the gaps and opportunities for health targets.
“Enabling healthy and nutritious foods for people and planet”
Can you share any upcoming activities of the Task Force?
We currently have two active expert groups, one working on the potential of probiotics during pregnancy, and the other on recommendations for designing appropriate clinical studies for probiotics research. We’ll soon launch an activity around markers of the gut microbiota and how those relate to human health. And an activity together with other ILSI branches around the world to investigate what consumers understand of postbiotics. This is another advantage of ILSI, having branches around the world that make it possible to address global topics.
Are there any specific probiotic strains or emerging applications that the Task Force is particularly excited about?
The Task Force’s work is precompetitive, so we do not look at specific strains or applications. We look at the larger picture. In that perspective, I am personally very interested in the upcoming work about consumers’ perspective on postbiotics. Marketing wise it may be challenging: for decades we have been telling consumers that beneficial microbes need to be alive to confer health benefits. With postbiotics, some may think ‘well, that viability does not really matter’. That is, of course, not the case. So, we must get the message right.
In your opinion, what role will probiotics play in shaping the future of health and nutrition?
I think we are only limited by our own imagination. When I started in this field 30+ years ago the idea that you could reduce respiratory tract infections by eating probiotics seemed absurd. Now it is one of the best documented health benefits. The gut-brain axis is now a well-established concept, there is great potential there. But there are many axes between the gut and other organ systems. Outside the field of nutrition, there are many other microbiota. Every surface of the body that is exposed to the environment has a microbiota; what can we do there? And of course, a natural extension are the live biotherapeutic products: ‘probiotic drugs’.
Finally, I want to say that I am only the chairman of the task force. I am deeply in debt to all present and past task force members, the external experts and the ILSI Europe staff I have had the privilege to work with in the task force. Without them sharing their knowledge, dedicating their scarce time, bringing their positive spirit and good humour (yes, we do have fun!), nothing of this would have been achieved. THANK YOU!
Can you elaborate on the applications of Biova's ingredients?
Biova's egg membrane-derived ingredients find applications in several key areas:
- Nutraceuticals: One of the primary applications of Biova's ingredients is in the dietary supplements market. Our ingredients are used in products designed to support joint health, skin & hair health, and overall wellness.
- Cosmetics and Personal Care: Biova's ingredients are utilized in the cosmetic industry, particularly in skincare products. They can be used in formulations aimed at improving skin elasticity, hydration, and overall appearance.
- Pet Nutrition: Biova also extends its ingredient applications to the pet supplement market, offering products that support joint health and mobility in animals.
How does Biova ensure the quality and safety of its ingredients throughout the process, from collecting egg membranes to producing the final water-soluble powdered product?
Biova is in a unique position when it comes to quality assurance and controls. We work with some of the largest egg breaking facilities in the USA. We are vertically integrated with the egg breaking facilities and collect the membranes on site at the breaker. The eggs are broken, and the shells move directly into our patented equipment for membrane removal, before being taken to our plant for further processing.
We have strict controls in place to ensure quality and safety of our ingredients. Additionally, we undergo voluntary audit by the FDA and USDA each year to ensure the highest manufacturing standards.
References and notes
1. Żmuda P et al. Bioavailability of Liposomal Vitamin C in Powder Form: A Randomized, Double-Blind, Cross-Over Trial. Appl Sci. 2024;14(17):7718.


