KEYWORDS
Probiotics
Prebiotics
Immunity
Microbiota
Secretory immunoglobulin A
Abstract
The pandemic thrust the immune market to the forefront, with consumers seeking clinically validated solutions to stay healthy or at least lessen the symptoms and severity of illness. Dietary components may help or hinder the development of certain microorganisms and contribute to the overall composition of one's gut microbial genetic pool, which can heavily impact the body’s immune response. Two major components that play a role in the gut's microbial diversity are probiotics and prebiotics. Innovative new approaches to clinical study have validated two distinct categories: pro- and prebiotics; in the case of our studies, probiotics such as the strain CNCM i-2745 aids in maintaining sufficient levels of critical immune system antibodies, while the yeast cell wall from Saccharomyces cerevisiae prebiotic induce the production of short-chain fatty acids, known to contribute to enhancing the immune response.
Introduction
Eating a varied nutrient-rich diet and living a healthy lifestyle are required for the health and function of all cells, including immune cells. Each stage of the body's immune response relies on the presence of many micronutrients, which help the immune system in several ways: working as an antioxidant to protect healthy cells, supporting the growth and activity of immune cells, and producing antibodies.
Ingredients like vitamins D, C, and B and minerals such as zinc have proven themselves in immune choices (1). Still, consumers seek innovative ingredients with clinical evidence and demonstrated efficacy.
Enter the biotics! The gut microbiome significantly impacts one’s health, and scientists are just now beginning to understand all its complexities. Decades of research have shown that the microbiome and the gut’s microbial diversity can be modulated with many distinct pro- and prebiotics (2).
Probiotics: The immune-ingredient leader
Probiotics occupy an important place in the competitive market of immunity ingredients and are now perceived as a clear solution for consumers. According to a 2020 survey from the Council for Responsible Nutrition (CRN), probiotics are in the top five ingredients dedicated to immunity. In 2022, US consumers showed a higher interest in probiotics, ranking even higher than zinc (1).
Let’s first discuss some immunity basics. Antibodies called immunoglobulin drive an essential part of immune health. An antibody is a protein secreted by the immune system in response to the presence of pathogen substances, the antigens. Antibodies will detect antigens and destroy them via the macrophages.
One of the most critical antibodies of the immune system is the secretory immunoglobulin A (sIgA). This antibody is in the mucosa, the most vulnerable interface with the environment. The mucosal surfaces, such as the linings of the respiratory and gastrointestinal tracts, represent an enormous area of potential exposure to pathogens. The sIgA as an antibody can catch toxins and infectious agents and inhibit the interaction with the respiratory and intestinal epithelium. The effect of sIgA on pathogens can be considered the body’s first line of defense (3).
Some factors influence the level of sIgA in the human body. The first is age; as we age, the level of sIgA decreases. Some other external factors can lead to a decrease in the sIgA concentration, such as intensive training, long-term use of medication, or even our modern lifestyle, which can leave us stressed, fatigued, and sleep-deprived. All these different factors directly impact our immune system, weakening our natural defenses (3).
Consumers are now aware that peak performance requires maintaining a healthy and powerful immune system, which means keeping a high level of sIgA. To that end, LifeinU® BSCU1 has been recently shown (3) to be a unique probiotic clinically validated to impact immunity. This probiotic is a specific strain of Bacillus subtilis, a common and safe bacterium registered in the CNCM collection under the number I-2745.
Efficacy validated by clinical study
To investigate the immunity benefits of this specific strain of B. subtilis, a double-blind, randomized, placebo-controlled clinical trial was initiated in collaboration with an independent expert in Gastroenterology and Immunology: Professor Philippe Marteau from Paris 7 university and AP-HP, Hôpital Saint Antoine, Paris, France.
The 4-month clinical study saw 100 volunteers with a history of common infections split into two groups: 50 supplemented with the strain of B. subtilis and 50 with placebo every first 10 days of each month.
At the end of the study, the level of sIgA of the supplemented group was higher compared to the placebo group: by 45% in saliva, and by 65% in the intestine, after 10 days of consumption, and by up to 87% increase compared to placebo at the end of the study (3).
Further, this clinical study also investigated symptoms, something of high importance to consumers. It showed that supplementation reduced the frequency of upper respiratory tract infections by 45% (4). Moreover, in the supplemented group, researchers observed a reduction of the average number of days of infectious episodes by 38% (2.8 days less compared to the placebo group) (4). The percentage of people who experienced one or more infectious episodes was also reduced by 31% (3).
An innovative scientific approach
To further investigate the benefits of this unique strain of B. subtilis, a new in-vitro model, representing a pioneering scientific standard, was recently developed. Not only can it be adapted to different ingredients and allows the avoidance of animal models, making it more ethical, but it mimics as close as possible the human model in the preclinical stage to anticipate the results that may be observed during the clinical trials (3).
The new model, called the “intestine-chip,” is a 3D human-printed model. Cells from the intestine are collected and cultivated to recreate a cellular microenvironment like the human one. The top part is equivalent to the lumen of the intestine with the epithelial cells, the bottom part is composed of endothelial cells that mimic blood circulation. With this organization, the environment is dynamic because some flow can be implemented in the top or bottom channels. This innovation aims to apply immune cells to the endothelial channel (unpublished results).
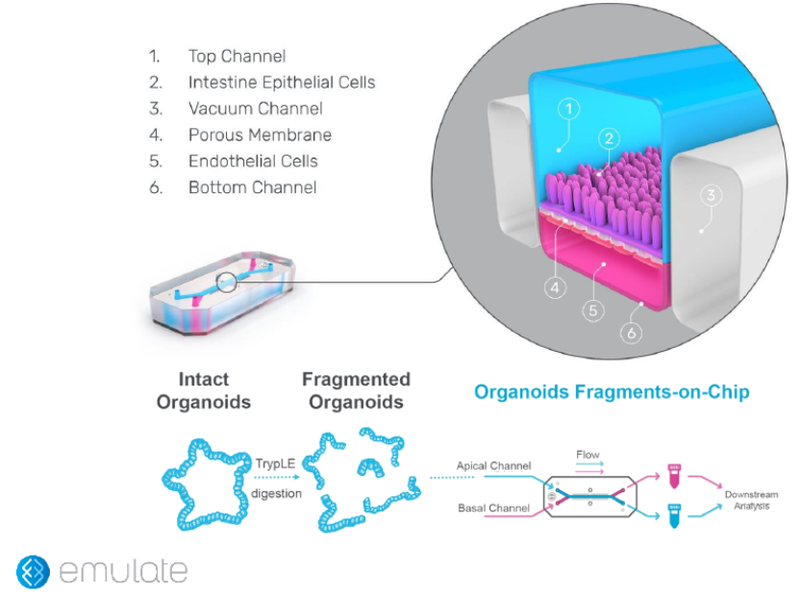
Supplementation with the unique strain of B. subtilis in this model highlights an increased concentration of the sIgA, in correlation with the results observed during the clinical trial. These results again confirm the positive impact on sIgA concentration and immune health (unpublished results).
Prebiotics: Immunity improvement through microbiota diversity
Prebiotics are mainly carbohydrate compounds that can be found in food, such as fruits, vegetables, whole grains, and yeasts. They are selectively fermented by beneficial bacteria within the gut microbiota, resulting in health benefits (5).
This fermentation allows bacteria to thrive, producing short-chain fatty acids (SCFA) as part of their metabolic activities. Among those metabolites, butyrate and propionate, for example, are known to be involved in the proliferation and differentiation of immune cells. Meanwhile, acetate regulates the pH of the gut to protect against pathogenic bacteria (6).
Well-known prebiotics in the market include Inulin, Galacto-oligosaccharides (GOS), and Fructo-Oligosaccharides (FOS). They are known to be effective with at least 5g per day, which unfortunately can cause bloating and flatulence. Further, historical prebiotics stimulate the same families of bacteria, namely Lactobacilli and Bifidobacteria (7).
But knowing that the gut contains thousands of billions of microorganisms classified in around 100 to 400 families, why restrict to so few different prebiotics?
Answering the needs for bacteria diversity
Beyond this broad bacteria population, we all share a common structure in our microbiota composition. Yet everyone displays their own microbiota signature, and similar compositions can be gathered in three main microbiota profiles: the enterotypes.
Each enterotype is relatively linked to geographical areas and thus to lifestyle and food consumption habits. The enterotypes are identified by the dominance of members from one or another bacteria family and by specific metabolic activity.
Since the metabolic activities are selective, it is important to offer a wide spectrum of prebiotics to achieve microbiome diversity. We all have thousands of probiotics to feed (and all those probiotics do not have all the same enzymes materials to fermentate the same kind of prebiotics). Depending on the enterotype to which we belong, this probiotic population will not be exactly the same in terms of proportion, hence even more important to have adapted prebiotic intake.
A loss of diversity is generally accepted due to time and societal changes. Less fermented food, less whole grain, and increased food preservation are potential factors that may have progressively decreased the richness of the human microbiota (8).
Nutrition intervention is a means to play on the microbiome diversity. Providing personalized micronutrition is a great way to encourage each bacterial family to thrive in its own unique microbiota environment.
To that end, Gnosis by Lesaffre launched the first patented yeast-based prebiotic (Lynside® Immunity Prebiotic). Indeed, the yeast cell wall part of this Saccharomyces cerevisiae offers a complex structure that is degraded by only a few fundamental bacteria species not usually targeted by other prebiotics (4). It stimulates different bacteria family depending on the host enterotype.
Ongoing unpublished studies on a dynamic in-vitro gut model are supporting the hypothesis of a long-lasting fermentation of the yeast-based prebiotic in proximal and distal colons. These results seem to show that it induces both an adapted metabolic activity and a modulation of the microbiota composition, depending on the host enterotype. If these findings are confirmed, this scenario may promote, combined with a very low daily dosage requirement, a continued prebiotic response with low gas production, avoiding the current prebiotic side effects.
Conclusion
The gut microbiome heavily impacts the body’s ability to protect itself and the world of biotics offers a wealth of new opportunities for the immunity market. As cutting-edge validation methods, such as the "intestine-chip” model, re being introduced, there is great potential for the dietary supplement and functional foods industries to support the immune response with probiotics, whose impact is emphasized by prebiotics.
References and notes
- Council for Responsible Nutrition report “Supplemental Security Income”, 2020, Unlocking Insights into the Supplement Consumer: 2022 Trends and Opportunities - Market Place
- Cunningham M et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021 Aug; 29(8): 667-685.
- Lefevre M, et al. Probiotic strain LifeinU™ BSCU1 stimulates immune system of elderly during common infectious disease period: a randomized, double-blind placebo-controlled study. Immun Ageing. 2015 Dec 3;12:24.
- Rodriguez B. A yeast product, and a composition comprising it, for use as a prebiotic agent. Patent WO2020152229. 2020.
- Gibson, G., Hutkins, R., Sanders, M. et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14, 491–502 (2017). https://doi.org/10.1038/nrgastro.2017.75
- Belkaid Y. et al. Role of the microbiota in immunity and inflammation. Cell. 2014 Mar 27; 157(1): 121-141.
- Markowiak P and Slizewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017 Sep; 9(9): 1021.
- Sonnenburg E.D. et al. Starving our Microbial Self: The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates. Cell Metabolism. 2014 Nov 4; 20(5):779-786.



