KEYWORDS
Microbiome
Gut microbiome
Healthy ageing
Telomere
Dysbiosis
Irritable bowel syndrome
Abstract
In the past century, overall lifespan has significantly increased, and health span is a growing area of interest to keep later years active, playful, and physically and cognitively independent. Maintaining an optimal gut ecosystem has a strong potential to contribute to a better quality of life with age. The gut microbiota and its metabolites modulate various functions in the human body. Our current understanding indicates a vital role of gut microbes in pathogenesis of diseases that affect a healthier lifespan. The composition and diversity of gut microbiota is altered with ageing. Gut dysbiosis has the potential to interrupt the communication between the microbiota and the host and trigger inflammaging, increasing the risk of various health disorders and mortality. Current evidence suggests the possibility of modulation of gut microbiota by several factors including diet and physical activity to delay age-related disorders.
Introduction
Currently, adults 60 years of age or more outnumber children under 5 years of age, and by 2050 adults ages 60+ are expected to outnumber adolescents and young people between the ages of 15 and 24 (1). As the pace of ageing population continues to accelerate, the United Nations General Assembly has declared 2021–2030 as the “Decade of Healthy Ageing.” Healthy ageing is defined as developing and maintaining the functional ability that enables well-being in older age (1). Ageing is inevitable and happens at both cellular and molecular levels. It is a complex process and manifests itself differently in different individuals. Human lifespan and health span are determined by various factors, including genetics, epigenetics, diet, and lifestyle (2). There is accumulating evidence suggesting a healthy lifestyle in early years is critical to maintaining health and curtailing the risk of age-related disorders later in life.
The human gut microbiome is increasingly being studied as an important contributing factor of healthy ageing due to its key role in both physical and mental health. The human microbiome is a complex ecosystem, and due to its critical role in human biology, it is sometimes referred to as a separate virtual organ. A healthy gut microbiota plays a key role in overall health and its microbial diversity is linked to several human diseases. A higher microbial diversity in the gut is associated with a healthier microbiota (3) while low diversity is linked to various disorders including obesity and gastrointestinal pathologies like inflammatory bowel disease (4). The diversity of microbes can be defined as the number and distribution of distinct types of organisms within the body. The gut microbiota of the elderly differs in composition and diversity from young and middle-aged adults (5) and this shift is described as one of the primary causes of ageing-related disorders. The composition and diversity of gut microbiota is influenced by several factors, such as diet, physical activity, geography, and general lifestyle.
Impact of diet and physical activity on microbiome and ageing
The human microbiome has a symbiotic relationship with its host. Diet is a critical modifiable factor for optimal health. Adherence to a healthy and balanced diet plays a major role in maintaining a lower frailty index. Diet is also a known modulator of gut microbiota, its composition, diversity, function, and metabolism activity (6). The overall output of microbial metabolites is significantly dependent on the intake of dietary components. The microbial metabolites include short chain fatty acids (SCFAs), neuroactive molecules, vitamins, epigenetic factors, hormones, neurotransmitters, and probably hundreds of other unidentified factors (7). These metabolites are known to influence both local intestinal tissues and peripheral tissues via various bidirectional communications referred to as gut-brain axis, gut-muscle axis, and gut-skin axis to name a few (8). It is estimated that approximately 10% of energy from diet is obtained due to gut microbial fermentation and subsequent short chain fatty acid production (9).
There is a linear relation between physical activity and health of an individual. Physical activity in older age may not only help prevent and mitigate falls, but also health conditions like sarcopenia, osteoporosis, and cognitive impairment, promoting healthy ageing (10). Studies indicate a bidirectional relationship between exercise and the gut microbiota composition (8). Exercise during older age can also assist with optimal composition of the gut microbiota in addition to improving cardiovascular fitness (11).
Microbiota in health and disease
Changes throughout the lifespan
Core gut microbiota is known to change during old age. Increase in biological age results in a reduction in overall gut microbiota richness, while advancing chronological age increases phylogenetic richness with more diverse gut microbiota (12). Measuring biological age has been proposed as an important factor in ageing. Figure 1 depicts the changes in host when the core microbiota shifts (12). When biological age is used with adjustment for chronological age, the microbiota richness decreases and results in increased gut dysbiosis and ultimately unhealthy ageing (12). During ageing, there is an attenuation of Bifidobacterium and Lactobacilli species which are crucial in health maintenance (13). Reduced abundance of healthy microbes may open space for opportunistic pathobionts. Akkermansia, Bifidobacterium, and Christensenellaceae in the gut have been associated with longevity (14).
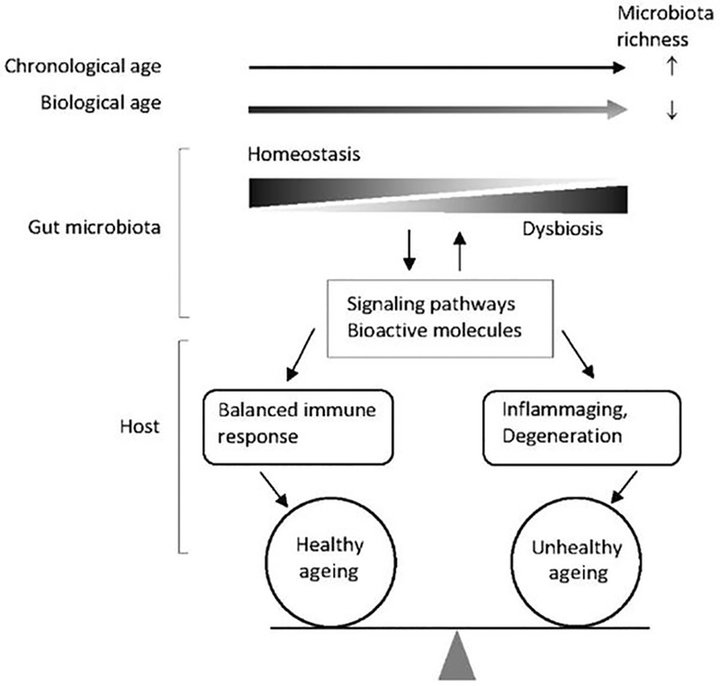
Figure 1. Increase in biological age is associated with reduced microbiota richness, dysbiosis and can prevent healthy ageing (12).
Gut dysbiosis
The gut microbiome and its metabolites that act as cofactors in various pathways can directly influence epigenetics (15). Microbial dysbiosis, which is a result of altered microbiota, impacts ageing and is linked to various diseases. A few of those maladies include obesity (16), diabetes (17), cancers (18), inflammatory diseases (19), and cardiovascular diseases (20). It is also implicated in the diseases of the central nervous system such as Parkinson’s (21). A decrease in Bifidobacteria has been postulated as one of the prominent factors in a variety of health disorders (22) suggesting its importance in health. Today, Bifidobacterium probiotics are widely used for health management through dysbiosis rectification. Obesity can cause inflammation and impair the intestinal epithelial integrity by shifting the microbial composition in the gut (23). The microbial changes in obesity are also known to occur in ageing and are the prime cause of various pathologies and disorders including frailty, diabetes, cancer, cardiovascular disease, and neurodegeneration (23).
Immunity and inflammaging
It is believed that the development of immune functions and gut microbiota may be mutually dependent and influenced by each other. The microbiota is fundamental in induction, training, and function of the immune system , and this starts at birth (24). Early life immune-microbiota interactions have long-lasting impact on health later in life (25). The strong link between gut microbiome and immune system has already formed the basis of microbiome-mediated therapeutic strategies in immune-mediated diseases. For example, fecal microbiome transplantation has been used as a potential treatment to restore a healthy microbiome in Clostridium difficile infections (25).
Age related immune dysfunction or immunoscence compromises both innate and adaptive immune responses, which play a primary role in chronic low-grade inflammation or inflammaging (26). This is associated with elevated pro-inflammatory cytokines that lead to a higher risk of morbidity and mortality. They are also indicative of various organ specific, age-related physical and cognitive disorders such as sarcopenia, osteoporosis, type 2 diabetes, atherosclerosis, dementia, Alzheimer’s disease, and Parkinson’s disease (27,28). Interleukin-6 (IL-6) is the proinflammatory cytokine that is most associated with ageing. It is significantly increased in the elderly (29) due to aged biome and accounts for increased susceptibility to infections and various phenotypic changes of ageing (30).
Mental and cognitive health
There is mounting evidence implicating the role of gut microbiota in central nervous system disorders. Gut dysbiosis is associated with a decreased level of brain-derived neurotrophic factor (BDNF), which can be regulated by butyrate, a metabolite of gut microbiota (31). Reduced levels of BDNF are directly linked to depression and brain stress (32). Impaired microglia cells are linked to neurodegeneration and several central nervous system disorders (33). Responsible for regulating brain development, maintaining neural network, and repairing injuries, they are pivotal in neuroinflammation modulation (34). Animal studies have demonstrated regulation of microglia cells by the gut microbiota, where age-related impaired gut barrier can influence microglia dysfunction (35). Chronic inflammation, which is influenced by the gut microbiota, is also considered as an important risk factor in the pathogenesis of mental disorders (36). Imbalance in the gut microbiota is associated with impaired cognition (37). The microbiome-gut-brain-axis may be able to explain the pathogenesis of various psychiatric disorders including depression (31). It is one of the most studied pathways that highlights the constant communication between the gut and the brain to modulate cognitive function in ageing. Cognition is important for functional independence, especially in older age. Cognitive abilities are believed to weaken with age. Based on current cumulative evidence, it can be determined that a healthy gut microbiota may potentially play a role in decreasing the rate of age-related cognitive decline.
Telomeres and microbiota
Ageing is also linked to the length of telomeres. Telomeres are at the end of chromosome and are vital in its structure and function. With each cell division, telomeres shorten in length and progressively shortening telomeres are linked to activation of DNA damage and cell senescence. The rate of telomere shortening may suggest the rate of ageing (38). Diet and lifestyle are well studied factors that impact telomeres (38). Similar to the microbial diversity and population, there are various factors that are implicated in negatively impacting the telomeres, such as obesity (38), stress (39), depression (40), smoking (41), environmental pollution (42), and gut dysbiosis (43), thus directly affecting the rate of ageing.
Targeting telomere shortening in the gut during ageing may potentially be a strategy to extend life and health span (44). Telomerase is an enzyme that can counter telomere shortening (44). Expression of telomerase in the gut to counteract systemic ageing has been successfully studied in zebrafish. Not only was telomerase able to reduce inflammation to restore a healthy gut environment, but its positive impact was also extended to other organs via the gut (44). Physical activity and exercise have been strongly connected to preserved telomeres (45). Exercise increases the biodiversity of gut microbiota and reduces telomere attrition in addition to decreasing inflammation and oxidative stress, further highlighting the interconnectedness of these factors in health and ageing (46). Antioxidants can help prevent oxidative damage that leads to telomere shortening (49). A diet rich in vitamins C and E and beta-carotene is linked to longer telomeres (47).
In addition, omega 3 fatty acid consumption is associated with reduced telomere attrition (48). Omega 3 fatty acids have also been found to significantly impact the intestinal environment and gut microbiota composition. These polyunsaturated fatty acids have shown a positive influence on the gut-brain axis, acting through gut microbiota composition (49).
Conclusion
The microbial ecosystem is fundamental to life and health. Ageing is largely associated with inflammation and immune system dysfunction. The ability of gut microbiota to influence proinflammatory cytokines to control inflammation indicates its critical role in ageing. The gut microbial diversity decreases with ageing, contributing to various age-related disorders. This directly implicates the role of gut microbiota in a longer lifespan. A compromised intestinal barrier has a negative effect on nutrient absorption and pathogen defense, both of which can lead to an array of health disorders. Gut dysbiosis disrupts the microbial communication with the host, which is associated with both age- related physical and cognitive health decline. It is also implicated in telomere shortening, thus negatively impacting longevity.
Based on our current scientific understanding, there is an untapped potential in the human gut. There is substantial data that suggest that our intestinal microbiota can be exploited to promote health, hence the rapidly advancing commercially available biotics. Continued clarity of the microbiome and its overall impact on the host and vice versa will present various opportunities to shape host health and design effective therapeutic strategies, potentially for a longer life and health span. A lot of questions remain unanswered: What does the “core set” of microbiota in its healthiest form look like? Can the microbiome be successfully modulated early in life to maximize chances of a healthy and disease-free life? This begs the question, does the answer to healthy ageing lie in a healthy ageing gut?
References and notes
- UN Decade of healthy ageing: Plan of action - world health organization [Internet]. [cited 2023 Jun 20]. Available from: https://cdn.who.int/media/docs/default-source/decade-of-healthy-ageing/decade-proposal-final-apr2020-en.pdf?sfvrsn=b4b75ebc_28
- Govindaraju D, Atzmon G, Barzilai N. Genetics, lifestyle and longevity: Lessons from Centenarians. Applied & Translational Genomics. 2015;4:23–32. doi:10.1016/j.atg.2015.01.001
- Yu X, Wu X, Qiu L, Wang D, Gan M, Chen X, et al. Analysis of the intestinal microbial community structure of healthy and long-living elderly residents in Gaotian village of Liuyang City. Applied Microbiology and Biotechnology. 2015;99(21):9085–95. doi:10.1007/s00253-015-6888-3
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and Lean Twins. Nature. 2008;457(7228):480–4. doi:10.1038/nature07540
- Ragonnaud E, Biragyn A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immunity & Ageing. 2021;18(1). doi:10.1186/s12979-020-00213-w
- Sonnenburg JL, Bäckhed F. Diet–microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64. doi:10.1038/nature18846
- Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends in Neurosciences. 2016;39(11):763–81. doi:10.1016/j.tins.2016.09.002
- Marttinen M, Ala-Jaakkola R, Laitila A, Lehtinen MJ. Gut microbiota, probiotics and physical performance in athletes and physically active individuals. Nutrients. 2020;12(10):2936. doi:10.3390/nu12102936
- Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiological Reviews. 1990;70(2):567–90. doi:10.1152/physrev.1990.70.2.567
- Eckstrom E, Neukam S, Kalin L, Wright J. Physical activity and healthy aging. Clinics in Geriatric Medicine. 2020;36(4):671–83. doi:10.1016/j.cger.2020.06.009
- Morita E, Yokoyama H, Imai D, Takeda R, Ota akemi, Kawai eriko, et al. Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients. 2019;11(4):868. doi:10.3390/nu11040868
- Kim S, Jazwinski SM. The gut microbiota and Healthy Aging: A mini-review. Gerontology. 2018;64(6):513–20. doi:10.1159/000490615
- Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, et al. Gut Microbiota and extreme longevity. Current Biology. 2016;26(11):1480–5. doi:10.1016/j.cub.2016.04.016
- Assis V, de Sousa Neto IV, Ribeiro FM, de Cassia Marqueti R, Franco OL, da Silva Aguiar S, et al. The emerging role of the aging process and exercise training on the crosstalk between gut microbiota and telomere length. International Journal of Environmental Research and Public Health. 2022;19(13):7810. doi:10.3390/ijerph19137810
- Hullar MA, Fu BC. Diet, the gut microbiome, and Epigenetics. The Cancer Journal. 2014;20(3):170–5. doi:10.1097/ppo.0000000000000053
- Delzenne NM, Cani PD. Interaction between obesity and the gut microbiota: Relevance in nutrition. Annual Review of Nutrition. 2011;31(1):15–31. doi:10.1146/annurev-nutr-072610-145146
- Yang Q, Lin SL, Kwok MK, Leung GM, Schooling CM. The roles of 27 genera of human Gut Microbiota in ischemic heart disease, type 2 diabetes mellitus, and their risk factors: A Mendelian randomization study. American Journal of Epidemiology. 2018;187(9):1916–22. doi:10.1093/aje/kwy096
- Dart A. Gut microbiota bile acid metabolism controls cancer immunosurveillance. Nature Reviews Microbiology. 2018;16(8):453–453. doi:10.1038/s41579-018-0053-9
- Hakansson A, Molin G. Gut microbiota and inflammation. Nutrients. 2011;3(6):637–82. doi:10.3390/nu3060637
- Jie Z, Xia H, Zhong S-L, Feng Q, Li S, Liang S, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nature Communications. 2017;8(1). doi:10.1038/s41467-017-00900-1
- Clairembault T, Leclair-Visonneau L, Coron E, Bourreille A, Le Dily S, Vavasseur F, et al. Structural alterations of the intestinal epithelial barrier in parkinson’s disease. Acta Neuropathologica Communications. 2015;3(1). doi:10.1186/s40478-015-0196-0
- Arboleya S, Watkins C, Stanton C, Ross RP. Gut Bifidobacteria populations in human health and aging. Frontiers in Microbiology. 2016;7. doi:10.3389/fmicb.2016.01204
- Ragonnaud E, Biragyn A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immunity & Ageing. 2021;18(1). doi:10.1186/s12979-020-00213-w
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–41. doi:10.1016/j.cell.2014.03.011
- Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Research. 2020;30(6):492–506. doi:10.1038/s41422-020-0332-7
- Lee K-A, Flores RR, Jang IH, Saathoff A, Robbins PD. Immune Senescence, immunosenescence and aging. Frontiers in Aging. 2022;3. doi:10.3389/fragi.2022.900028
- FRANCESCHI C, BONAFÈ M, VALENSIN S, OLIVIERI F, DE LUCA M, OTTAVIANI E, et al. Inflamm-aging: An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2006;908(1):244–54. doi:10.1111/j.1749-6632.2000.tb06651.x
- Ventura MT, Casciaro M, Gangemi S, Buquicchio R. Immunosenescence in aging: Between immune cells depletion and cytokines up-regulation. Clinical and Molecular Allergy. 2017;15(1). doi:10.1186/s12948-017-0077-0
- Nicoletti C. Age-associated changes of the intestinal epithelial barrier: Local and systemic implications. Expert Review of Gastroenterology & Hepatology. 2015;9(12):1467–9. doi:10.1586/17474124.2015.1092872
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annual Review of Medicine. 2000;51(1):245–70. doi:10.1146/annurev.med.51.1.245
- Suda K, Matsuda K. How microbes affect depression: Underlying mechanisms via the gut–brain axis and the modulating role of Probiotics. International Journal of Molecular Sciences. 2022;23(3):1172. doi:10.3390/ijms23031172
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nature Neuroscience. 2007;10(9):1089–93. doi:10.1038/nn1971
- Ransohoff RM, Khoury JE. Microglia in health and disease. Cold Spring Harbor Perspectives in Biology. 2015;8(1). doi:10.1101/cshperspect.a020560
- Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annual Review of Immunology. 2017;35(1):441–68. doi:10.1146/annurev-immunol-051116-052358
- Bostick JW, Mazmanian SK. Impaired gut barrier affects microglia health. Nature Neuroscience. 2022;25(3):268–70. doi:10.1038/s41593-022-01028-2
- Alam R, Abdolmaleky HM, Zhou J-R. Microbiome, inflammation, epigenetic alterations, and mental diseases. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2017;174(6):651–60. doi:10.1002/ajmg.b.32567
- Koblinsky ND, Power KA, Middleton L, Ferland G, Anderson ND. The role of the gut microbiome in diet and exercise effects on cognition: A review of the intervention literature. The Journals of Gerontology: Series A. 2022;78(2):195–205. doi:10.1093/gerona/glac166
- Shammas MA. Telomeres, lifestyle, cancer, and aging. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14(1):28–34. doi:10.1097/mco.0b013e32834121b1
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences. 2004;101(49):17312–5. doi:10.1073/pnas.0407162101
- Kinser PA, Lyon DE. Major depressive disorder and measures of Cellular Aging: An Integrative Review. Nursing Research and Practice. 2013;2013:1–10. doi:10.1155/2013/469070
- Valdes A, Andrew T, Gardner J, Kimura M, Oelsner E, Cherkas L, et al. Obesity, cigarette smoking, and telomere length in women. The Lancet. 2005;366(9486):662–4. doi:10.1016/s0140-6736(05)66630-5
- Hoxha M, Dioni L, Bonzini M, Pesatori AC, Fustinoni S, Cavallo D, et al. Association between leukocyte telomere shortening and exposure to traffic pollution: A cross-sectional study on traffic officers and indoor office workers. Environmental Health. 2009;8(1). doi:10.1186/1476-069x-8-41
- Bazaz MR, Balasubramanian R, Monroy-Jaramillo N, Dandekar MP. Linking the triad of telomere length, inflammation, and gut dysbiosis in the manifestation of depression. ACS Chemical Neuroscience. 2021;12(19):3516–26. doi:10.1021/acschemneuro.1c00457
- El Maï M, Bird M, Allouche A, Targen S, Şerifoğlu N, Lopes-Bastos B, et al. Gut-specific telomerase expression counteracts systemic aging in telomerase-deficient zebrafish. Nature Aging. 2023;3(5):567–84. doi:10.1038/s43587-023-00401-5
- Schellnegger M, Lin AC, Hammer N, Kamolz L-P. Physical activity on telomere length as a biomarker for aging: A systematic review. Sports Medicine - Open. 2022;8(1). doi:10.1186/s40798-022-00503-1
- Assis V, de Sousa Neto IV, Ribeiro FM, de Cassia Marqueti R, Franco OL, da Silva Aguiar S, et al. The emerging role of the aging process and exercise training on the crosstalk between gut microbiota and telomere length. International Journal of Environmental Research and Public Health. 2022;19(13):7810. doi:10.3390/ijerph19137810
- Shen J, Gammon MD, Terry MB, Wang Q, Bradshaw P, Teitelbaum SL, et al. Telomere length, oxidative damage, antioxidants and breast cancer risk. International Journal of Cancer. 2009;124(7):1637–43. doi:10.1002/ijc.24105
- Farzaneh-Far R. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303(3):250. doi:10.1001/jama.2009.2008
- Costantini L, Molinari R, Farinon B, Merendino N. Impact of omega-3 fatty acids on the gut microbiota. International Journal of Molecular Sciences. 2017;18(12):2645. doi:10.3390/ijms18122645



