KEYWORDS
Upcycling
Olive
Cardiovascular health
Cholesterol
Carbon footprint
Irritable bowel syndrome
Abstract
The beneficial role of extra-virgin olive oil consumption is well established and often associated to a lower incidence of CVD, thanks to its strong antioxidant capacity. The present work aims at presenting the evidence of the effectiveness of a new food supplement ingredient, based on highly standardized polyphenols from olive oil by-products, targeting hypercholesterolemia conditions.
Derived from a circular economy involving only Mediterranean olive by-products, it is dedicated to the control of unbalanced levels of cholesterol, leading to satisfactory benefits in reducing oxidative stress and systemic inflammatory levels. A series of in-vitro and clinical tests will be described as a support of the scientific background of the ingredient.
Introduction
According to the WHO, cardiovascular disease (CVD) globally causes around 17.9 million deaths per year (1). A combination of different metabolic comorbidities such as obesity, impaired glucose tolerance, and hypertriglyceridemia can affect the development of cardiovascular disorders. Therefore, the higher risk of CVD is more related to a syndrome than to a single factor (2).
Metabolic syndrome (MetS) is in fact defined as a cluster of interconnected factors that directly increase the risk of coronary heart disease, in addition to other forms of atherosclerotic cardiovascular diseases and type 2 diabetes. It is mainly characterized by at least three concomitant distinctive signs including dyslipidemia, high blood pressure, dysregulated glucose homeostasis, insulin resistance, and abdominal obesity. It has a complex and multifaceted origin; however, sedentarism and unbalanced dietary patterns might play a fundamental role in its development (3).
The risk factors associated with the aetiology of CVD and MetS are many; some are of genetic origin, while others may be subjected to modulation: among them, for example, blood pressure, cholesterol, lifestyle and diet. Mediterranean diet, in particular, plays an important role in the prevention of such diseases since it is rich in unsaturated and saturated fatty acids: the correct proportion of these two lipid molecules is the key to reach and maintain a healthy lifestyle (4,5). One of the protagonists in the Mediterranean diet is olive: its derivative, the olive oil, represents the primary source of dietary fatty acids and a great source of antioxidants (6). The main phytochemicals identified and quantified in olives and olive oil are phenolic and non‐phenolic compounds, with hydroxytyrosol and tyrosol representing the most abundant compounds of the phenolic class. (7,8).
In 2011, the European Food Safety Authority (EFSA) published a health claim related to polyphenols in olive oil and their possible protection of blood lipids against oxidative stress, stating that 5 mg of hydroxytyrosol and its derivatives (e.g., oleuropein complex and tyrosol) should be consumed daily in the context of a balanced diet for the sufficient avoidance of oxidative damage (9,10). Olive hydroxytyrosol acts in fact as a free‐scavenger and reduces the levels of low‐density lipoprotein cholesterol (LDL‐C) oxidation, platelet aggregation, and chronic inflammation. In this way, it helps counteract atherosclerosis‐related cardiovascular diseases (11,12).
The development
From the historical expertise of ROELMI HPC at the service of the Life Science sector, with its entrepreneurial roots in the long Mediterranean tradition, and from the spirit of sustainable innovation and deep technological knowledge, SelectSIEVE® OptiChol has been developed.
It is a natural active ingredient intended for nutraceutical applications for the promotion of a healthy cardiovascular system, through the balance of cholesterol levels by decreasing LDL and boosting HDL.
Based on balanced and highly standardized polyphenols from olive (Olea europaea), this is the result of the company’s proactive commitment to eco-responsible phytocomplexes, developed through modern extraction processes, which allow not only to preserve the properties of natural active ingredients contained in the leaves of the selected plants, but also to promote the Company's commitment to environmental sustainability, through the setting up of a circular economy focused on the enhancement of plant matrices.
Derived from a circular economy involving only olive by-products of the variety Coratina, an Apulian native cultivar, the development of the ingredient has demonstrated the possibility of ennobling a by-product coming from Italian agro-food industry into a resource of great, social and environmental, as well as economic value.
The sustainable features
The production of olive oil in Italy is a traditional activity, result of a millennial expertise (13) including specific processing techniques mainly divided into 5 phases. The activity starts with the harvesting to collect the product followed by the washing where the olives are weighed and subjected to an abundant washing in water before a pressing process. The next operation is represented by the kneading whichconsists of a continuous and slow mixing of the dough for the extraction of the oil from the olive paste. This phase can be performed with different techniques: pressure, centrifugation, or percolation. The final phase is the separation of the olive oil from the water thanks to the centrifugal force.
The vegetation water resulting from the pressing of olives can be considered a by-product of the normal production of olive oil and it is rich in bio-actives molecules (about 3-5kg per ton of water), i.e. polyphenols with antioxidant and antibacterial properties. Therefore, due to this peculiar composition, such water cannot be released into the environment or used for agriculture, but it requires preliminary purification treatments. These treatments are dedicated to produce pure water that can be returned to the environment and sludge used for the production of biogas as well as the isolation of the polyphenols fraction of from the rest.
The technology used for the recovery of vegetation water, already known for the water purification itself, has been revisited here in a circular key, to further improve the selection steps of the isolated fractions, in order to obtain a high added-value ingredient, reproducible, certified for food grade and effective in controlling of cholesterol levels.
The selected supply chain for the production of the ingredient relies on certified organic suppliers to obtain safe vegetation water that does not contain pesticides or pollutants. Production also takes place in a plant very close to olive oil mills, to make the supply chain short and enhance the local resources of the South.
The industrialization of the production process and the marketability of the ingredient also demonstrate the technical and economic feasibility of the project.


Efficacy dossier (14)
A clinical trial was carried out to evaluate the effect of the nutraceutical active ingredient on the plasma cholesterol of hypercholesterolemic individuals. The study was designed as a single‐arm, non‐controlled, non‐randomized, prospective pilot clinical study, and it involved a sample of 30 Italian volunteers recruited from the Lipid Clinic of the S. Orsola‐Malpighi University Hospital (Bologna, Italy) among patients referring for polygenic hypercholesterolemia.
Participants were required to be aged 20–70 years, with LDL‐C > 115 mg/dL and < 190 mg/dL and not undergoing a lipid‐lowering treatment. After a 2‐week period of diet standardization with low‐fat and low‐sodium Mediterranean diet, the enrolled subjects were instructed to take a capsule/day, before breakfast, of the nutraceutical active ingredient which contained 100 mg of olive‐derived extract standardized in hydroxytyrosol with acacia gum and maltodextrin as inactive carriers. The intervention period lasted 4 weeks. Before and after the dietary supplementation with the nutraceutical active ingredient, patients were evaluated for their clinical status and by the execution of laboratory analyses. At the end of the study, all of the unused capsules were retrieved for inventory, and the participants’ compliance was assessed by counting the number of returned capsules.
The main objective of the study was to measure through a simple blood analysis the total cholesterol (TC), high‐density lipoprotein cholesterol (HDL-C), Non‐HDL cholesterol (Non‐HDL‐C), low-density lipoprotein cholesterol (LDL‐C), triglycerides (TG), together with serum uric acid (SUA), creatinine, fasting plasma glucose (FPG), alanine transaminase (ALT), aspartate transaminase (AST), gamma‐glutamyl transferase (gGT), and glomerular filtration rate (eGFR). All these parameters were considered indicative to highlight a potential effect of the study product in improving the plasma cholesterol of the enrolled subjects.
Additionally, arterial blood pressure (BP) was also assessed, as resting systolic (SBP) and diastolic BP (DBP) in order to determine the food supplement’s benefits in terms of arterial hypertension.
Lastly, a Food Frequency Questionnaire (FFQ) was used to assess the demographic variables of smoking, dietary habits, leisure time and physical activities among participants. Safety and tolerability were continuously monitored throughout the study in order to detect any adverse event. Patients’ acceptability of the nutraceutical active ingredient was also measured with a 10-point visual analogue scale (VAS).
Regarding the results of the study, the nutraceutical active ingredient was able to modulate of the whole lipidemic profile and related dyslipidemia parameters by downregulating total cholesterol, LDL cholesterol and non-HDL cholesterol, together with an increase of HDL cholesterol. It also demonstrated activity in reducing other risk factors of cardiometabolic disease by improving systolic blood pressure and pulses, reducing fasting plasma glycaemia and decreasing serum uric acid (Table 1).
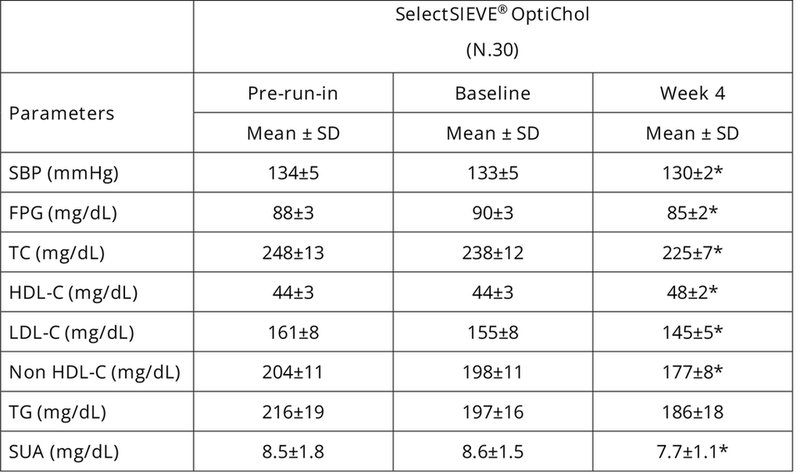
Table 1. Hemodynamic and blood chemistry parameters from the baseline to the end of the clinical trial. SBP= Systolic Blood Pressure, FPG= Fasting Plasma Glucose, TC= Total Cholesterol, HDL-C= High Density Lipoprotein Cholesterol, LDL-C= Low-Density Lipoprotein Cholesterol, TG= Triglycerides, SUA= Serum Uric Acid.
*p<0.05 vs. baseline
Moreover, all participants completed the study according to its design (dropout rate = 0%) and they all were compliant with the protocol. No statistically significant changes were recorded in dietary habits during the study, with no changes in the total energy and in the macronutrient intake. Finally, volunteers’ acceptability of the nutraceutical active ingredient was good and the treatment was generally well tolerated by the subjects without any reported adverse event as well as any occurred laboratory abnormality.
Therefore, the effectiveness of the nutraceutical active ingredient has been clinically proven, since it could represent an aid for the treatment of moderate hypercholesterolemia regulating the total amount of blood cholesterol together with an improvement of all cardiometabolic parameters involved in the aetiology of MetS.

Conclusion
The ingredient perfectly reflects the necessity to offer a valid ally in counteracting hypercholesterolemia condition. Its efficacy is proven in different in-vitro models (data not shown) as well as on humans. Together with its efficacy, the sustainable origin is also an important feature by enhancing food by-products into nutraceutical ingredients.
Thanks to a well-accomplished mechanism of action, the ingredient demonstrates a cholesterol-lowering effect making it suitable for the development of food supplements supporting cardiovascular well-being.
References and notes
- 1.WHO, 2021
- Tune JD, Goodwill AG, Sassoon DJ, Mather KJ. Cardiovascular consequences of metabolic syndrome. Transl Res. 2017 May;183:57-70. doi: 10.1016/j.trsl.2017.01.001. Epub 2017 Jan 9. PMID: 28130064; PMCID: PMC5393930.
- Bovolini A, Garcia J, Andrade MA, Duarte JA. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int J Sports Med. 2021 Mar;42(3):199-214. doi: 10.1055/a-1263-0898. Epub 2020 Oct 19. PMID: 33075830.
- Beulen, Y.; Martínez‐González, M.A.; van de Rest, O.; Salas‐Salvadó, J.; Sorlí, J.V.; Gómez‐Gracia, E.; Fiol, M.; Estruch, R.; Santos‐Lozano, J.M.; Schröder, H.; et al. Quality of Dietary Fat Intake and Body Weight and Obesity in a Mediterranean Population: Secondary Analyses within the PREDIMED Trial. Nutrients2011, 10. https://doi.org/10.3390/nu10122011.
- Guasch‐Ferré, M.; Babio, N.; Martínez‐González, M.A.; Corella, D.; Ros, E.; Martín‐Peláez, S.; Estruch, R.; Arós, F.; Gómez‐Gracia, E.; Fiol, M.; et al. Dietary fat intake and risk of cardiovascular disease and all‐cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr.2015, 102, 1563–1573.
- Nan, J.N.; Ververis, K.; Bollu, S.; Rodd, A.L.; Swarup, O.; Karagiannis, T.C. Biological effects of the olive polyphenol, hydroxytyrosol: An extra view from genome‐wide transcriptome analysis. Hell. J. Nucl. Med.2014, 17, 62–69.
- Uylaşer, V.; Yildiz, G. The historical development and nutritional importance of olive and olive oil constituted an important part of the Mediterranean diet. Crit. Rev. Food Sci. Nutr.2014, 54, 1092–1101. https://doi.org/10.1080/10408398.2011.626874.
- Rocha, J.; Borges, N.; Pinho, O. Table olives and health: A review. J. Nutr. Sci. 2020, 9, e57. https://doi.org/10.1017/jns.2020.50.
- Rizwan, S.; Benincasa, C.; Mehmood, K.; Anjum, S.; Mehmood, Z.; Alizai, G.H.; Azam, M.; Perri, E.; Sajjad, A. Fatty Acids and Phenolic Profiles of Extravirgin Olive Oils from Selected Italian Cultivars Introduced in Southwestern Province of Pakistan. J.Oleo. Sci.2019, 68, 33–43. https://doi.org/10.5650/jos.ess18150.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (Id 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (Id 1639), maintenance of normal blood pressure (Id 3781), “anti‐inflammatory properties” (id 1882), “contributes to the upper respiratory tract health” (Id 3467) pursuant to article 13(1) of regulation (ec) no 1924/2006. EFSAJ. 2011, 9, 2033.
- KarkovićMarković, A.; Torić, J.; Barbarić, M.; JakobušićBrala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules2019, 24, 2001. https://doi.org/10.3390/molecules24102001.
- Vilaplana‐Pérez, C.; Auñón, D.; García‐Flores, L.A.; Gil‐Izquierdo, A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front. Nutr.2014, 1, 18. https://doi.org/10.3389/fnut.2014.00018.
- Caracuta, V. Olive growing in Puglia (southeastern Italy): a review of the evidence from the Mesolithic to the Middle Ages. Veget Hist Archaeobot 29, 595–620 (2020). https://doi.org/10.1007/s00334-019-00765-y
- 14.Cicero, A.F.G.; Fogacci, F.; Di Micoli, A.; Veronesi, M.; Grandi, E.; Borghi, C. Hydroxytyrosol-Rich Olive Extract for Plasma Cholesterol Control. Appl. Sci. 2022, 12, 10086. https://doi.org/10.3390/app121910086


