Consumer perspective
— Column
Personalised interventions linking genetics and lifestyle to address obesity
KEYWORDS
Obesity
Polygenic background
Lifestyle
Personalised Interventions
Artificial intelligence
Digestive health
Immune health
Immune health
Obesity is a complex disorder influenced by genetic, cardiometabolic, lifestyle, and environmental factors. Personalized interventions targeting genetics and lifestyle are a promising strategy, but precisely understanding the molecular basis of gene-gene and gene-environment interactions remains a challenge due to the complex nature of obesity. In this article we provide an overview of the genetic, nutrigenetic, and lifestyle factors contributing to the rising global prevalence of obesity and overweight. It also introduces future directions, including the BETTER4U project, which aims to address this issue by employing machine learning, AI, and modern monitoring tools to create and test personalized recommendations for weight management. This approach further clarifies the interactions of genetics, omics, and lifestyle, potentially improving the success of obesity interventions.
Introduction
Obesity and overweight constitute an increasingly alarming global public health concern, due to a dramatic rise in their prevalence during the past several decades. It is estimated that in the next 20 years, almost one-quarter of the global adult population will suffer from obesity, leading to crises in healthcare systems, with severe consequences in groups from multiple socioeconomic statuses (1) . Despite the substantial progress in clinical studies in the field, a shared accepted approach in their prevention and treatment has yet to be agreed, rendering the need for effective strategies an ongoing public health emergency.
Obesity, in itself, is a multifactorial disease determined by factors of genetic makeup, cardiometabolic profile (i.e. glycaemic and lipidemic profile), lifestyle determinants (for example smoking, dietary habits, physical activity regimen) and environmental factors (i.e. geographic and contextual parameters). Considering these factors determine obesity for an individual of any age, its onset heavily relies on the interplay between genetic liability, circumstances surrounding birth and early-life and the impact of the current obesogenic environment (2,3). Heritability studies have demonstrated that obesity-predisposing genes can interact with a variety of environmental and lifestyle exposures, contributing to the observed variation in obesity levels between individuals and populations (4). These gene-environment interactions are further supported by evidence from monogenic and polygenic obesity studies, which have identified specific genetic variants that confer susceptibility to obesity in the presence of certain environmental factors (4). Addressing both genetic background and lifestyle behaviour, thus, seems to be the key toward long-term success in the prevention and management of overweight problems and related disorders of cardiometabolic nature.
In this context, this review is aimed at providing an overview of some of the most relevant and recent genetic, nutrigenetic and lifestyle findings, as well as personalised approaches to obesity prevention and treatment, with potential for use in clinical practice.
Understanding the genetic basis of obesity
Research in the field of genetics has contributed to a better understanding of the molecular mechanisms underlying obesity manifestation. Genetic background can have a direct impact in the manifestation of pathogenic obesity, such as in the cases of syndromic and monogenic obesity, or a more complex influence, as in the case of polygenic obesity which is attributed to the cumulative impact of multiple genetic compounds. The genetic architecture of increased body weight has been the subject of many genome-wide association studies (GWAS) during the past years, with significant findings putting genetics to the forefront of obesity etiology (5) (e.g. the identification of specific variants explaining BMI variance).
Genetic determinants are considered to be the primary factor responsible for the variation in adiposity within increasingly obesogenic environments. GWAS over the past decades have identified several hundreds of genetic loci harbouring variants that contribute to obesity onset. While a few of the genes located in the proximity of the identified loci harbour mutations with very strong effects on adiposity and disease (i.e. FTO and MC4R genes) (6,7), a multitude of alleles with smaller effect sizes in or close to genes involved in neural signalling, adipogenesis, and energy metabolism capture most of the genetic variance in obesity. As in other complex diseases, effectively capturing the overall impact of genetic makeup dictates the need for methodologies focusing on the aggregated effect of multiple loci instead of the unique influence of several – albeit important – single nucleotide polymorphisms (SNPs).
A polygenic risk score (PRS) quantifies an individual's genetic susceptibility to obesity by aggregating the effects of multiple genetic variants using GWAS data. PRSs calculating the genetic risk for obesity onset can be used as risk prediction tools to identify individuals at higher risk and allow for early preventative interventions. Although PRSs are influenced by population specificity and interpretation limitations, they hold the potential to be incorporated in clinical settings as a way to leverage genetic information in the daily practice (8-10).
Impact of lifestyle and environmental factors on obesity
It is known that obesity and associated diseases are strongly influenced by genetic, epigenetic, and environmental factors, such as lifestyle and diet. Currently, there is a greater awareness among consumers regarding health issues, with an increasing ability to decide which medical tests to perform. However, similarly to the progress in genomics, behaviour and lifestyle should also be considered when choosing a more effective solution.
The rise in obesity prevalence was primarily attributed to an imbalance in the energy intake and expenditure, resulting from the overlapping influence of increased exposure to high-energy nutrient-dense food and a sedentary lifestyle. Over the past six decades, increased obesity rates have paralleled increased portions and high consumption of sugar-rich and processed foods. The additional lack of regular physical activity can further contribute to the accumulation of body fat, with special attention to visceral fat associated with numerous metabolic disorders, including type 2 diabetes and cardiovascular disease. Consequently, disrupted energy balance and insufficient exercise not only promote weight gain but also exacerbate the health risks linked to obesity (11, 12).
Integration of genetics and lifestyle in personalised obesity management
Future directions and implications
Acknowledgements
Additional lifestyle and behavioural characteristics can affect obesity parameters both directly and indirectly. For example, stress and/or inadequate or poor-quality sleep disrupts the balance of appetite-regulating hormones and can lead to a vicious cycle of poor dietary choices and metabolic disturbances (13). To boot, factors of environmental or contextual character also have a direct impact on weight management, such as aspects of the built environment (i.e. urban design and transportation), socioeconomic characteristics (income, education and work environment) and cultural norms (14,15).
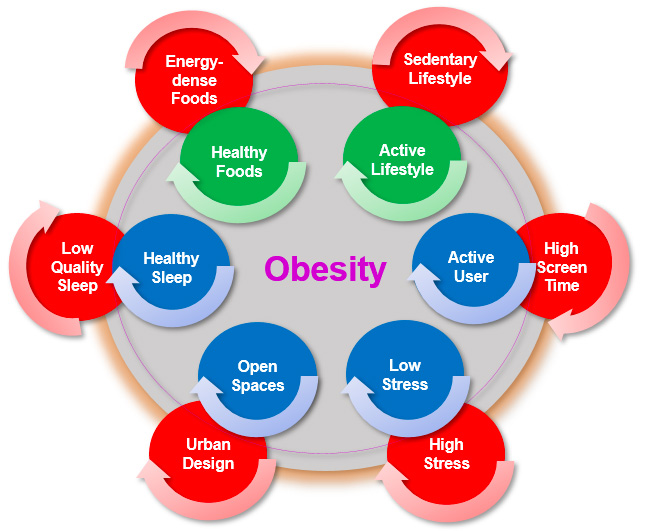
Figure 1. Lifestyle Components related to Obesity.
Figure 2. Multidisciplinary Integration of Obesity-related Components
Personalised interventions targeting obesity by linking genetics and lifestyle represent a cutting-edge approach to tackling a matter as complex as the management of increased body weight. Since obesity-related comorbidities cannot be prevented without weight control, management, and reduction or the use of disease-specific interventions across the entire obesity spectrum, lifestyle remains the key approach to obesity prevention and treatment (16). Among commonly employed interventions, while lifestyle has remained as the cornerstone, technologically-assisted, personalised programs with genotype information to optimize clinical success are regarded as one promising strategy for better prevention and treatment outcomes (17).
Methodologies pertaining to personalization embrace the linkage of genetics to lifestyle to treat patients with obesity with tailored lifestyle programs essential to harness the power of genetic information in obesity parameters. Genotype information alone is usually insufficient to modify disease risks owing to an inability to conclude disease mechanisms and then develop the directed strategies. A necessary issue that needs to be underscored is identifying the most proficient and safest lifestyle interventions to modulate the pathogenesis of obesity or its related comorbidities and integrating genotype-derived information to guide the individualized approach. However, precisely understanding the molecular basis of the gene-gene and gene-environment interactions becomes impeded due to the complex nature of common disease, thus a lifestyle-genetic-risk-prediction score model including both genetic and lifestyle information remains the priority (18).
Lifestyle-oriented strategies leverage the unique genetic makeup of individuals and their specific lifestyle factors to create tailored prevention and treatment plans using genotype information, clinical and other lifestyle components. The implementation strategy of such approaches entails: i) the comprehensive assessment of the individual, including genetic testing and evaluation of lifestyle habits and health status; ii) the setting of specific, measurable, achievable, relevant and time-based goals (i.e. SMART) goals for the individuals progress of modifications in weight or behaviour-related parameters; iii) the implementation of an interdisciplinary approach with behavioural and lifestyle interventions, including personalised recommendations on dietary and exercise plans and use of modern monitoring tools to evaluate adherence and progress, and; iv) the continuous adaptation of the SMART goals based on the individual’s dynamic feedback and potential new insights (19).
Success of obesity interventions has been limited by the wide range of responses to available interventions of behavioural, dietary, activity and pharmacological character. Precision prescription of lifestyle and drug interventions will improve their performance and limit use of unnecessary, often unsuccessful and expensive interventions selected by trial and error, providing actionable recommendations for obesity prevention and treatment. Modelling and functional characterization of obesity-predisposing and protective GWAS variants, genes, omics (20) and regulatory networks using perdurance of genomics databases incorporated in system biology and in silico models, can lead to personalised medicine insights, have potential for discovery of other complex traits and will assist in accelerating clinical care.
In this context, BETTER4U is a multidisciplinary EU project, precisely aiming at understanding the very origins of obesity onset. BETTER4U aspires to undertake all aforementioned questions regarding the insight on the variability of factors affecting obesity manifestation, their respective interplay and the optimal ways to modify them and halt weight gain. The project will employ machine learning, artificial intelligence methodology and modern monitoring tools to create and test personalised recommendations for weight management in adults with overweight and obesity. BETTER4U will also further clarify the interactions of genetic, omics and lifestyle compounds in the effort to address obesity across the spectrum of age and socioeconomic background.
For more information on the BETTER4U project please visit www.better4u.eu and/or contact Professor Georgios Dedoussis.
This article was written by the authors on behalf of the BETTER4U Consortium Members.
Parents understand that the maintenance of their child’s health and wellness is their responsibility; however, many parents face various challenges when trying to do so. FMCG Gurus findings highlight that sugar is the ingredient that parents are most conscious about in food and drink products, with 74% of consumers concerned by sugar content in products. As children are typically drawn to sugary indulgences, parents are concerned by the link between obesity and diabetes and the hidden sugars in products.
Many parents believe that the complex labeling used by brands disguises ingredients. As a result, brands should ensure that nutritional labeling is made clear and simple for parents so that they are able to unpick the nutritional profile of products within seconds.
References and notes
- Janssen F, Bardoutsos A, Vidra N. Obesity Prevalence in the Long-Term Future in 18 European Countries and in the USA. Obes Facts. 2020;13(5):514-527. https://doi.org/10.1159/000511023
- Diels, S., Vanden Berghe, W., & Van Hul, W. (2020). Insights into the multifactorial causation of obesity by integrated genetic and epigenetic analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity, 21(7), e13019. https://doi.org/10.1111/obr.13019
- Lin, X., & Li, H. (2021). Obesity: Epidemiology, Pathophysiology, and Therapeutics. Frontiers in endocrinology, 12, 706978. https://doi.org/10.3389/fendo.2021.706978.
- Reddon H, Guéant JL, Meyre D. The importance of gene-environment interactions in human obesity. Clin Sci (Lond). 2016;130(18):1571-1597. https://doi.org/10.1042/CS20160221.
- Locke, A. E., Kahali, B., Berndt, S. I., Justice, A. E., Pers, T. H., Day, F. R., Powell, C., Vedantam, S., Buchkovich, M. L., Yang, J., Croteau-Chonka, D. C., Esko, T., Fall, T., Ferreira, T., Gustafsson, S., Kutalik, Z., Luan, J., Mägi, R., Randall, J. C., Winkler, T. W., … Speliotes, E. K. (2015). Genetic studies of body mass index yield new insights for obesity biology. Nature, 518(7538), 197–206. https://doi.org/10.1038/nature14177.
- Fawcett KA, Barroso I. The genetics of obesity: FTO leads the way. Trends Genet. 2010;26(6):266-274. https://doi.org/10.1016/j.tig.2010.02.006
- Chami, N., Preuss, M., Walker, R. W., Moscati, A., & Loos, R. J. F. (2020). The role of polygenic susceptibility to obesity among carriers of pathogenic mutations in MC4R in the UK Biobank population. PLoS medicine, 17(7), e1003196. https://doi.org/10.1371/journal.pmed.1003196.
- Cross, B., Turner, R., & Pirmohamed, M. (2022). Polygenic risk scores: An overview from bench to bedside for personalised medicine. Frontiers in genetics, 13, 1000667. https://doi.org/10.3389/fgene.2022.1000667.
- Torkamani, A., & Topol, E. (2019). Polygenic Risk Scores Expand to Obesity. Cell, 177(3), 518–520. https://doi.org/10.1016/j.cell.2019.03.051.
- Kafyra, M., Dedoussis, G. V. Polygenic Risk Scores and Personalised Approaches to Cardiometabolic Disease Prevention and Treatment: A short review. J Atherosclerosis Prev Treat. 2023; Jan-Apr;14(1):35-43. https://doi.org/10.53590/japt.02.1045.
- Hwalla N, Jaafar Z. Dietary Management of Obesity: A Review of the Evidence. Diagnostics (Basel). 2020;11(1):24. Published 2020 Dec 25. https://doi.org/10.3390/diagnostics11010024.
- Raiman L, Amarnani R, Abdur-Rahman M, Marshall A, Mani-Babu S. The role of physical activity in obesity: let's actively manage obesity. Clin Med (Lond). 2023;23(4):311-317. https://doi.org/10.53590/japt.02.1045.
- Chaput, J. P., McHill, A. W., Cox, R. C., Broussard, J. L., Dutil, C., da Costa, B. G. G., Sampasa-Kanyinga, H., & Wright, K. P., Jr (2023). The role of insufficient sleep and circadian misalignment in obesity. Nature reviews. Endocrinology, 19(2), 82–97. https://doi.org/10.1038/s41574-022-00747-7.
- Congdon P. Obesity and Urban Environments. Int J Environ Res Public Health. 2019;16(3):464. Published 2019 Feb 5. https://doi.org/10.3390/ijerph16030464.
- Anekwe CV, Jarrell AR, Townsend MJ, Gaudier GI, Hiserodt JM, Stanford FC. Socioeconomics of Obesity. Curr Obes Rep. 2020;9(3):272-279. https://doi.org/10.1007/s13679-020-00398-7.
- Flint, S. W., & Batterham, R. L. (2023). The need to personalise approaches for the prevention and management of obesity. EClinicalMedicine, 58, 101944. https://doi.org/10.1016/j.eclinm.2023.101944.
- Lau, Y., Wong, S. H., Chee, D. G. H., Ng, B. S. P., Ang, W. W., Han, C. Y., & Cheng, L. J. (2024). Technology-delivered personalised nutrition intervention on dietary outcomes among adults with overweight and obesity: A systematic review, meta-analysis, and meta-regression. Obesity reviews : an official journal of the International Association for the Study of Obesity, 25(5), e13699. https://doi.org/10.1111/obr.13699.
- Holzapfel, C., Waldenberger, M., Lorkowski, S., Daniel, H., & Working Group “Personalised Nutrition” of the German Nutrition Society (2022). Genetics and Epigenetics in Personalised Nutrition: Evidence, Expectations, and Experiences. Molecular nutrition & food research, 66(17), e2200077. https://doi.org/10.1002/mnfr.202200077.
- Finn, E. B., Whang, C., Hong, P. H., Costa, S. A., Callahan, E. A., & Huang, T. T. (2023). Strategies to improve the implementation of intensive lifestyle interventions for obesity. Frontiers in public health, 11, 1202545. https://doi.org/10.3389/fpubh.2023.1202545.
- Woldemariam S, Dorner TE, Wiesinger T, Stein KV. Multi-omics approaches for precision obesity management : Potentials and limitations of omics in precision prevention, treatment and risk reduction of obesity. Wien Klin Wochenschr. 2023;135(5-6):113-124. https://doi.org/10.1007/s00508-022-02146-4.


