A ROLE FOR THE GUT MICROBIOTA AND THE PRECIBIOMIC STRAIN HAFNIA ALVEI HA4597 IN THE FIGHT AGAINST OBESITY

There is no need to remind how big of a public health issue obesity is globally, and despite the efforts from the WHO since the 1990s (1) and campaigns to eat less and move more in many countries, the prevalence of obesity and overweight keeps rising. The COVID-19 lockdowns only made matters worse, with a global weighted mean of 1.57 kg weight gain, equivalent to one-third point of BMI in the first lockdown (2). In France, a study conducted in May 2021 (3) showed that 55% of French people gained weight, with 6 kg on average! The WHO recently declared obesity to have reached epidemic proportions in Europe (4).
Doctors have even coined the new term “Covibesity” as early as June 2020 (5). Others warn of the upcoming epidemic of “depreobesity” (6). With a higher risk of getting COVID-19 for obese people, having more serious outcomes, and even an increased risk of death (7) the holistic understanding of health is gaining traction.
In this context, the past two decades’ discoveries around the gut microbiota’s role in metabolism and weight are offering hope. Human gut microbes were shown to be associated with obesity in 2006 (8) and soon after, the causative and transmissible role of microbes was described in two major studies. Microbiota transplantations from twins discordant for obesity – with fecal samples from obese women and their lean twin counterparts transferred to germ-free mice led the rodent recipients of the obese donors to become obese (9). Similarly, taking the human fecal microbiota from the same person before and after gastric bypass and transplanting it to mice showed that the obese phenotype pre-bypass is transmitted to the mice through the microbes (10). Several mechanisms involving calorie uptake, appetite regulation, fat mass regulation, and pro-atherosclerosis were identified (10, 11).
Many research projects and efforts focus on solutions to support weight management that stem from the microbiota: among them A-Mansia (12) with research on Akkermansia muciniphila, Ysopia Bioscience (13) with hopes to target obesity with Christensenella minuta as a drug, andTargEDys (14) with two decades of work to understand and leverage the role of microbes in appetite regulation, which now offers Hafnia alvei HA4597 as a weapon in the fight against obesity in several European countries (15). Go ahead and dig deeper into this exceptional scientific adventure.
In this context, the past two decades’ discoveries around the gut microbiota’s role in metabolism and weight are offering hope. Human gut microbes were shown to be associated with obesity in 2006 (8) and soon after, the causative and transmissible role of microbes was described in two major studies. Microbiota transplantations from twins discordant for obesity – with fecal samples from obese women and their lean twin counterparts transferred to germ-free mice led the rodent recipients of the obese donors to become obese (9). Similarly, taking the human fecal microbiota from the same person before and after gastric bypass and transplanting it to mice showed that the obese phenotype pre-bypass is transmitted to the mice through the microbes (10). Several mechanisms involving calorie uptake, appetite regulation, fat mass regulation, and pro-atherosclerosis were identified (10, 11).
Many research projects and efforts focus on solutions to support weight management that stem from the microbiota: among them A-Mansia (12) with research on Akkermansia muciniphila, Ysopia Bioscience (13) with hopes to target obesity with Christensenella minuta as a drug, andTargEDys (14) with two decades of work to understand and leverage the role of microbes in appetite regulation, which now offers Hafnia alvei HA4597 as a weapon in the fight against obesity in several European countries (15). Go ahead and dig deeper into this exceptional scientific adventure.
INTRODUCTION
Legrand et al. (21) tested Hafnia alvei HA4597 on two mice models of obesity: leptin-deficient Ob/Ob mice, and nutritionally induced obesity with a high-fat diet (HFD). The study confirmed that Hafnia alvei HA4597 significantly reduced body weight gain and fat mass in both models of obese mice while activating lipolysis. In leptin-deficient mice, the probiotic reduced food intake by over 20% in comparison to the control group, confirming the action through anorexigenic signaling pathways.
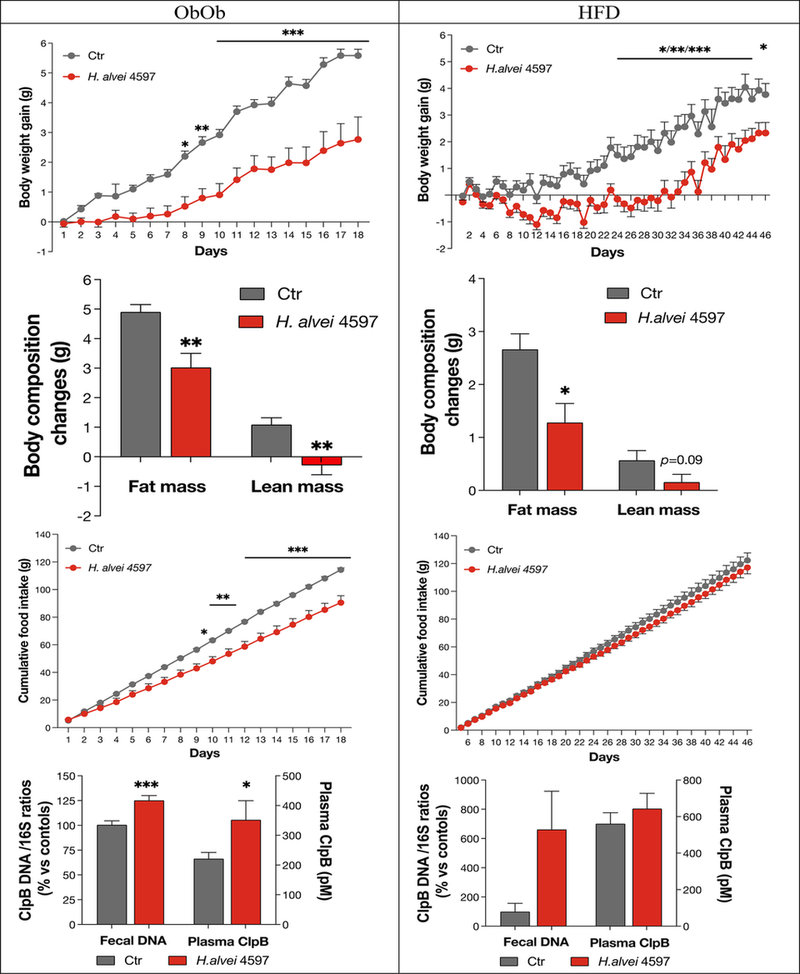
Figure 2. Effects of Hafnia alvei HA4597 and control in Ob/Ob mice and HFD mice (Legrand, 2020).
In another trial conducted by Lucas et al. (22), the efficacy of Hafnia was tested in a model of mice that were both hyperphagic (genetically leptin-deficient) and fed a high-fat diet, more representative of the schemes of excess eating combined with a hypercaloric diet in obese people, and even compared the strain’s effects to the drug Orlistat, a lipase inhibitor prescribed for the management of obesity. Hafnia alvei supplementation significantly reduced food intake, body weight gain, and total mass in these mice compared to the HFD control, resulting in an improved lean/fat mass ratio (Figure 2). The Orlistat-treated group was characterized by the lowest weight gain, but the drug led to a hyperphagic effect, with mice eating significantly more than in the high-fat diet alone, and marked by an increase in glycemia.
In addition, in the probiotic-supplemented group, metabolic benefits were observed, with improvements in basal glycemia and glycemic peak after a sugar challenge (Oral Glucose Tolerance Test or OGTT), compared to the HFD group and the Orlistat-treated mice.
PROOF OF EFFICACY IN ANIMALS
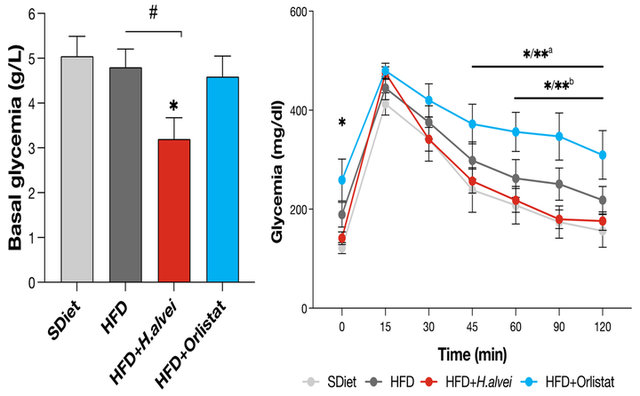
Figure 3. Glycemia and OGTT in Hafnia alvei HA4597 and Orlistat-treated mice (Lucas et al. 2020, 21).
Following the success of the preclinical studies, the evaluation of Hafnia alvei’s efficacy in supporting weight loss in humans was subsequently tested in 236 overweight adults in a 12-week prospective, multicentric, randomized, double-blind, placebo-controlled study (23). The participants were instructed to follow a 20% caloric restriction diet (with a tolerance for minus or plus 10% of calories) for 12 weeks while maintaining their usual level of physical activity. Half of the subjects received 2 capsules/day of Hafnia alvei, to be taken in the morning and at lunch at the dose of 100 billion cells/day and the other half received 2 capsules of placebo.
The primary endpoint was the percentage of responders, defined as people who lost a minimum of 3% of body weight in the 12-weeks period. Indeed, significantly more participants lost at least 3% of their initial weight in the probiotic group than in the placebo group (57.7% vs. 41.7%, p=0.028, + 38%). More participants also lost 4% of their body weight with HA than placebo (46.2% vs. 30.6%, P=0.024, +51%).
The participants in the probiotic group also had a steeper reduction in hip circumference and were feeling significantly less hungry than the placebo group as of 8 weeks into supplementation. In addition, blood parameters (glycemia, total cholesterol and LDL cholesterol) were significantly improved, even though these people were overweight but otherwise healthy. This is not surprising when considering that the global guidelines for obesity management mention that 3% to 5% weight loss is associated with meaningful clinical benefits: reductions in serum triglycerides concentrations, of blood glucose, HbA1C, and the risks of developing type 2 diabetes (24).
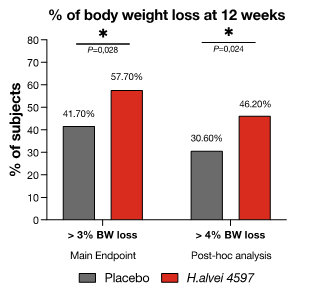
Figure 4. Effects of supplementation with Hafnia alvei and placebo for 12 weeks on the proportion of responders, losing at least 3% and 4% of body weight (Déchelotte, 2021, 23).
CLINICAL EFFICACY OF HAFNIA ALVEI HA4597 IN HUMANS
The medical practice of doctors, when faced with overweight and obese patients, is to recommend eating less and moving more. However, as well put by Dr. Arya Sharma, obesity management professor, and opinion leader, « Telling someone with obesity to eat less and move more is about as effective as telling someone with depression to cheer up ». People know what to do, but it is just so hard to comply, day after day, when they are hungry and yearning for food. Hafnia alvei HA4597 brings much-needed help to tune down hunger, to learn to pay better attention to satiety and fulness, and ultimately to lose weight while developing a better relationship with food.
WHY IS THIS IMPORTANT?
Nina Vinot has a background as a life sciences engineer from AgroParisTech and conducted nutrition research at Penn State University, CNRS and INRAe.
She has now 10 years experience promoting the use of beneficial bacteria, a track record of success developing sales across Europe for Probiotical, and is today international sales director for the French biotech TargEDys, pioneering precision probiotics.

20 years ago, at the Stockholm Karolinska Institute, neuroscientists Serguei Fetissov and Tomas Hökfelt discovered that patients with eating disorders like bulimia and anorexia displayed antibodies binding to anti-alpha-MSH, a key satiety hormone (16). Later, together with gastroenterologist Pierre Déchelotte in the prestigious laboratories of Inserm (the French National Institute for Medical Research), Dr. Fetissov was looking for the putative autoantigen present in microorganisms. Investigating gut bacteria using proteomics, they found a protein produced by Escherichia coli that presented a homology of sequence and conformation to alpha-MSH (17). This molecular mimetic was then identified as Caseinolytic peptidase B (ClpB) (18). Importantly, beyond its role as an antigen stimulating alpha-MSH receptors, ClpB was shown to directly activate satiety pathways including the release of satiety hormone PYY in the gut and activation of anorexigenic neurons in the brain (19, 20).
THE DISCOVERY
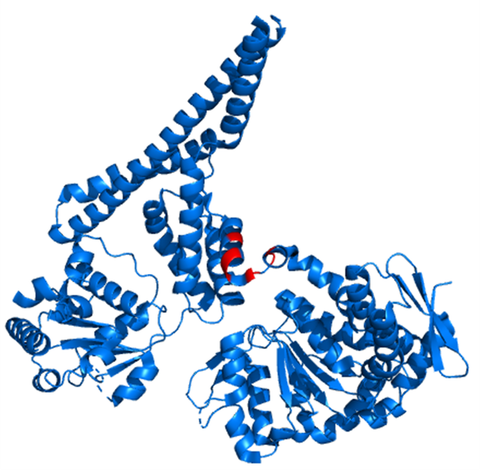
Figure 1. Caseinolytic peptidase B (ClpB) and its putative alpha-MSH-like pharmacophore (visible in red) displaying sequence and conformation homology to alpha-MSH.
Courtesy of Manon Dominique.
From there, the two researchers co-founded TargEDys to go beyond diagnostics and develop a safe and effective probiotic able to deliver ClpB and thus reduce appetite to support weight loss.
In 2014, investors supported the project and Gregory Lambert came on board as CEO and VP of R&D. He started searching for bacteria able to reach the market as probiotics. It was not an easy endeavor, because bacteria producing ClpB belong to the group of Enterobacteria, many of which are notorious for their pathogenicity and not listed on the EFSA Qualified Presumption of Safety. Although some E. coli strains had been used as a probiotic, other strains are very dangerous, such as enterohemorrhagic 0157:H7, so the market access for such bacteria was a long and winding road scattered with regulatory pitfalls.
Gregory found that one Enterobacterium, Hafnia alvei, was a key bacterium in several raw milk cheeses such as Camembert and Livarot and had been used since the early 90s as a starter culture to allow these cheeses to keep their organoleptic properties when made from pasteurized milk. This species had been first identified in 1954 by Moller in Copenhagen, and still bears its cradles’ name (Hafnia was the Latin name of Copenhagen). It has been described in different foods over the following decades and benefits from a long history of safe use, which has enabled its access to market before any other next-generation probiotic.
Hafnia alvei was indeed producing ClpB, in the same form as the one produced by E. coli, and in higher quantity (21). TargEDys then co-developed the industrial process with French fermenting company Bioprox to produce Hafnia in a powder form for a stable food supplement, and from there had the quantities needed to pursue the research.

MICROBIOME & WEIGHT MANAGEMENT




