INFANT NUTRITION
Health Claims for Infant Formula:
Advancing science-backed evidence and regulatory harmonization
Dr. Volker Spitzer1 , Dr. Stephanie Krammer-Lukas2 ,
Khee Suan Bang3 , Matt Stewart4
1. Vice President, Global R&D/RWE Services and Thought Leadership, IQVIA Consumer Health, Basel, Switzerland
2. Director, Global R&D/RWE Services and Thought Leadership, IQVIA Consumer Health, Basel, Switzerland
3. Associate Principal, Medical Affairs Strategy, Real World Solutions, IQVIA Consumer Health, Singapore
4. Global Marketing Manager, IQVIA Consumer Health, London, UK




KEYWORDS
Infant formula
Science-backed health claims
Marketing infant nutrition products
Ethical marketing
Digital responsibility
Abstract
Infant formula is essential when breastfeeding is not feasible or sufficient. Amid criticism of marketing strategies, a robust, science-based approach is needed to develop evidence on the health outcomes associated with infant formula intake. This article examines next steps to ensure progress in this complex area, including implementing ethical marketing efforts, enhancing research integrity, adopting innovative methods, promoting engagement with the public and other stakeholders, carrying out long-term studies, fostering collaborative research between academia and industry, and advocating for international standardization of regulations.
Introduction
Appropriate infant nutrition is essential for positive lifelong health outcomes. Infant formula plays a key role when breastfeeding is not feasible or sufficient. In the face of criticism of infant formula manufacturers’ marketing efforts, scientific evidence is needed to support the health benefits of these products. The clinical trials that can provide this evidence are challenging due to ethical and technical issues, including tracking dietary compliance and health outcomes. A rigorous scientific approach can ensure optimized study design, planning, implementation and regulatory oversight.

Optimum nutrition in infancy helps ensure growth and development, while reducing the risk of chronic diseases later in life (1). Benefits of breastfeeding are well documented for mothers and infants, with additional social, economic and environmental gains for communities. Yet, only 38% of infants worldwide are exclusively breastfed (1), and in the United States, only 24.9% are receiving breast milk exclusively at six months, as recommended by the American Academy of Pediatrics (2). Infant formula – which is designed to mimic human milk (3) – is intended to promote optimal growth, development, programming of the metabolic system and maturation of the immune system (4).
Sales of infant formula amount to some $55 billion/year worldwide, with China as the largest market (5). However, debate continues around marketing strategies and the quality of scientific evidence for health claims (6, 7, 8, 9, 10). The regulation of infant formula varies globally but aims to ensure truthful and scientifically substantiated health claims. The WHO's International Code discourages public promotion of infant formula to support breastfeeding, with 84 countries enacting this. In the United States, manufacturers must notify the FDA about new or significantly changed formulas and provide strong scientific evidence for compliance with nutritional standards, safety, and quality. The FDA can investigate and enforce action on misleading claims. In the European Union, new formulas must comply with EU regulations and EFSA guidance, with health claims banned since 2020 to encourage breastfeeding, except for compositional claims like DHA and reduced lactose. China has a rigorous process for health claims approval, with the National Food Safety Standard (GB 10765 – 2021) implemented in 2023, requiring scientific evidence for health claims. These appear in marketing materials or on product packaging, implying that the formula offers specific health or nutritional benefits. Examples include claims that the formula supports physical growth, strengthens the immune system, aids brain development, enhances visual acuity, supports digestive health, or lowers the risk of allergies. More general claims may also be made around the product benefits, such as usability; these should also be evidence-based, with research methods depending on the claim type.
These statements should be truthful and be underpinned by sound scientific evidence, such as results from clinical trials. Yet, varying regulations between countries reflect differing interpretations of what comprises sufficient evidence to support a health claim. This poses challenges for manufacturers, particularly in a global context with varying regulatory frameworks for manufacturing and marketing (11). Several global organizations support frameworks and standards for research on infant formula (12), but these guidelines are generally not legally binding and vary by country in adoption and enforcement.
A harmonized global approach – including standardized health and nutrition claims for infant formula – would inform a more consistent understanding of what each claim represents. This would require international cooperation between regulatory bodies, healthcare professionals and industries, including alignment of methodologies and health priorities. In turn, this harmonized approach would build consumer trust in the reliability and safety of infant nutrition products.
Building on the need for internationally aligned, science-based standards, it is essential to broaden our evidence spectrum—not only by adopting innovative digital data collection and adaptive study designs but also by integrating robust Real World Evidence (RWE) methodologies. RWE is increasingly recognized as a critical complement to traditional clinical trials in other areas and starts to play an important role in infant nutrition research as well. It offers broader insights into the long-term effects and practical use of infant formula across diverse populations. This approach not only overcomes some of the ethical and methodological constraints inherent in randomized trials but also enhances the generalizability of findings. Incorporating RWE into the evidence base can bridge gaps between controlled study environments and everyday practice.
By integrating a broader range of evidence methodologies tailored to address different aspects of infant nutrition claims—regulators could establish clearer, more precise evidence requirements. For instance, while controlled clinical trials remain ideal for substantiating immediate physiological effects, RWE is better suited to capture long-term outcomes and practical usage patterns. This comprehensive evidence framework enables a more nuanced assessment of health claims, thereby supporting regulatory frameworks that are robust, transparent, and harmonized across different markets.
Considerations for infant nutrition trials
Substantiating infant formula health claims through clinical studies is a complex effort, involving unique ethical, methodological, and regulatory challenges (13). The placebos often used in randomized drug trials cannot be used, since infants must be given complete nutrition to ensure their health and growth. Outcomes in formula studies usually involve milestones for growth and development, which depend on multiple factors. This makes it difficult to isolate the effects of the formula. Lastly, ambiguity poses a challenges for companies to navigate the regulatory landscape. Details of the challenges involved in infant formula trials are provided in Table 1.
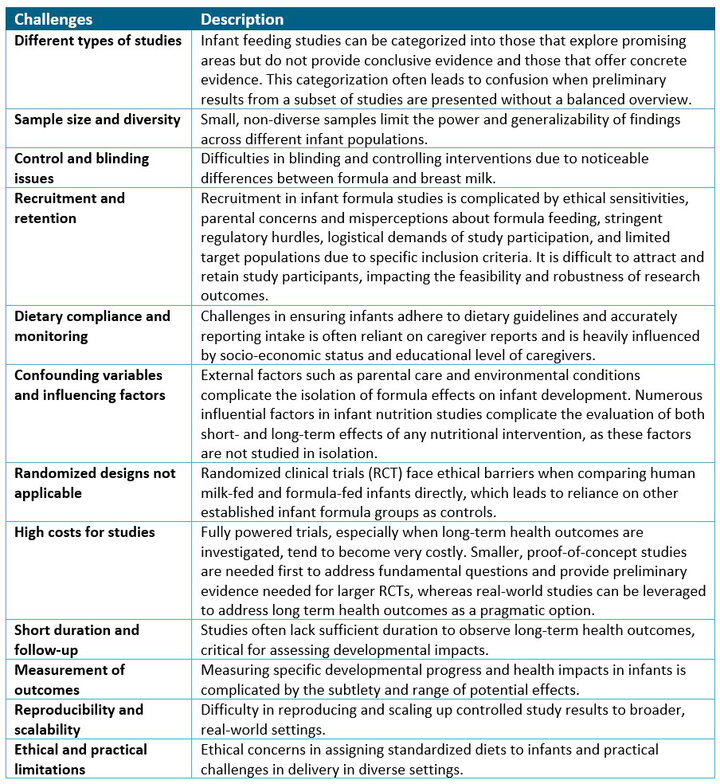
Table 1. Challenges frequently encountered in infant formula studies
Scientific opportunities and challenges in the personalized nutrition & health field
Areas for future efforts
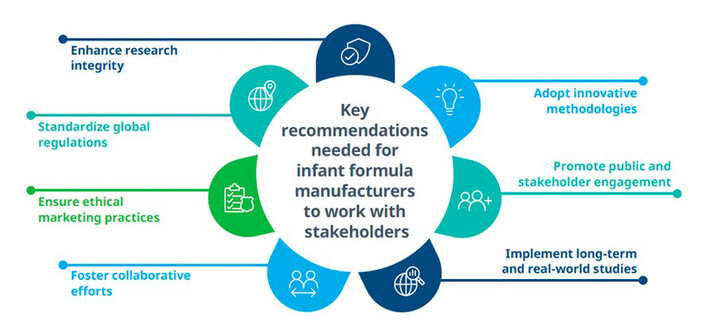
To advance evidence-based claims for infant nutrition products, and in turn to build public confidence, future efforts should focus on the following key areas:
- Implementing ethical marketing efforts that do not undermine or discourage breastfeeding, and help support informed decisions by providing validated scientific evidence
- Enhancing research integrity, including rigorous scientific validation of health claims and full reporting of trial results
- Adopting innovative methods to help overcome barriers to infant formula study participation, such as the use of digital tools for remote collection of data
- Promoting engagement of the public and other stakeholders to agree on public health goals, research objectives and to build trust
- Carrying out long-term and real-world studies to more fully understand health outcomes in formula-fed infants
- Fostering collaborative efforts to enhance infant nutrition research, including academia, healthcare organizations and industry
- Working towards standardizing regulations for infant formula, helping ensure clarity and consistency in health claims across countries
The future
Click to edit...
References and notes
- Martin CR, Ling PR, Blackburn GL. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients. 2016 May 11;8(5):279. doi: 10.3390/nu8050279. PMID: 27187450; PMCID: PMC4882692.
- Eidelman AI, Schanler RJ, Johnston M, Landers S, Noble L, Szucs K, Viehmann L. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841.
- U.S. Food and Drug Administration web page. Infant Formula. 2025Jan10.
- Kouwenhoven SMP, Muts J, Finken MJJ, Goudoever JBV. Low-Protein Infant Formula and Obesity Risk. Nutrients. 2022 Jun 30;14(13):2728. doi: 10.3390/nu14132728. PMID: 35807908; PMCID: PMC9268498.
- World Health Organization website. Marketing the US$55 billion formula milk industry.
- Rollins N, Piwoz E, Baker P, Kingston G, Mabaso KM, McCoy D, Ribeiro Neves PA, Pérez-Escamilla R, Richter L, Russ K, Sen G, Tomori C, Victora CG, Zambrano P, Hastings G; 2023 Lancet Breastfeeding Series Group. Marketing of commercial milk formula: a system to capture parents, communities, science, and policy. Lancet. 2023 Feb 11;401(10375):486-502. doi: 10.1016/S0140-6736(22)01931-6. Epub 2023 7 Feb. PMID: 36764314.
- Cheung KY, Petrou L, Helfer B, Porubayeva E, Dolgikh E, Ali S, Ali I, Archibald-Durham L, Brockway MM, Bugaeva P, Chooniedass R, Comberiati P, Cortés-Macías E, D’Elios S, Feketea G, Hsu P, Kana MA, Kriulina T, Kunii Y, Madaki C, Omer R, Peroni D, Prokofiev J, Simpson MR, Shimojo N, Siziba LP, Genuneit J, Thakor S, Waris M, Yuan Q, Zaman S, Young BE, Bugos B, Greenhawt M, Levin ME, Zheng J, Boyle RJ, Munblit D. Health and nutrition claims for infant formula: international cross sectional survey. BMJ. 2023 Feb 15;380:e071075. doi: 10.1136/bmj-2022-071075. PMID: 36792145; PMCID: PMC9930154.
- United Nations web page. Baby formula marketing ‘pervasive, misleading and aggressive’ – UN report, February 22, 2022.
- Morrison O. “The voluntary code is not working”: Experts call for an end to the ‘exploitative marketing’ used by the baby formula milk industry. FoodNavigator Europe. 2023Feb9.
- Munblit D, Crawley H, Hyde R, Boyle RJ. Health and nutrition claims for infant formula are poorly substantiated and potentially harmful. BMJ. 2020 May 6;369:m875. doi: 10.1136/bmj.m875. PMID: 32376671; PMCID: PMC8581741.
- Ruthsatz M. Foods for special medical purposes/medical foods: A global regulatory synopsis. RAPS Regulatory Focus. 2022Nov17.
- Codex Alimentarius. International Food Standards. Standard for infant formula and formulas for special medical purposes intended for infants. 2023.
- Szajewska H. Lessons learned from clinical studies on infant nutrition. World Rev Nutr Diet. 2013;106:3-11.doi: 10.1159/000342582. Epub 2013 Feb 11. PMID: 23428674.




